Osmosis Refers To The Movement Of
News Leon
Mar 26, 2025 · 6 min read
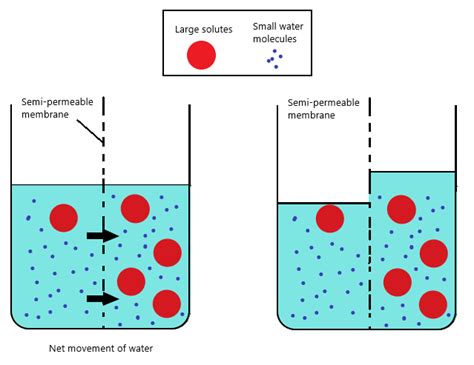
Table of Contents
Osmosis: A Deep Dive into the Movement of Water Across Membranes
Osmosis, a fundamental process in biology and chemistry, refers to the movement of water molecules across a selectively permeable membrane from a region of high water concentration to a region of low water concentration. This movement continues until equilibrium is reached, meaning the concentration of water is equal on both sides of the membrane. Understanding osmosis is crucial for grasping various biological processes, from plant growth to the function of our kidneys. This article will delve into the intricacies of osmosis, exploring its underlying mechanisms, significance in biological systems, and practical applications.
Understanding Selectively Permeable Membranes: The Gatekeepers of Osmosis
The cornerstone of osmosis is the selectively permeable membrane. This membrane acts as a barrier, allowing some substances to pass through while restricting others. In biological systems, the cell membrane is a prime example of a selectively permeable membrane. This membrane is composed primarily of a phospholipid bilayer, with embedded proteins that facilitate the passage of specific molecules. Water molecules, being relatively small and uncharged, can pass through the membrane via osmosis, while larger or charged molecules may require specific transport mechanisms.
The Role of Aquaporins: Facilitating Water Transport
While water can pass directly through the lipid bilayer, its movement is significantly enhanced by specialized protein channels called aquaporins. These integral membrane proteins form pores that allow water molecules to move across the membrane much more rapidly than they would otherwise. Aquaporins are highly selective, ensuring that only water molecules pass through, preventing the passage of other small molecules or ions. Their presence significantly impacts the rate of osmosis, particularly in cells where rapid water transport is essential.
Osmotic Pressure: The Driving Force Behind Osmosis
The driving force behind osmosis is osmotic pressure. This is the pressure required to prevent the net movement of water across a selectively permeable membrane. Osmotic pressure is directly proportional to the concentration of solute particles in a solution. A solution with a high concentration of solute particles will exert a higher osmotic pressure than a solution with a low concentration of solute particles. This pressure difference drives the movement of water from the region of lower solute concentration (higher water concentration) to the region of higher solute concentration (lower water concentration).
Isotonic, Hypotonic, and Hypertonic Solutions: Understanding Osmotic Relationships
To understand osmotic pressure better, it’s vital to grasp the concepts of isotonic, hypotonic, and hypertonic solutions. These terms describe the relative concentrations of solutes in two solutions separated by a selectively permeable membrane.
-
Isotonic solution: In an isotonic solution, the concentration of solutes is equal on both sides of the membrane. Therefore, there is no net movement of water across the membrane; the rate of water movement in both directions is equal.
-
Hypotonic solution: A hypotonic solution has a lower concentration of solutes (and therefore a higher concentration of water) than the solution on the other side of the membrane. Water will move from the hypotonic solution into the hypertonic solution, causing the hypotonic solution's volume to decrease and the hypertonic solution's volume to increase. This is often seen in plant cells, where water enters the cell, causing it to become turgid.
-
Hypertonic solution: A hypertonic solution has a higher concentration of solutes (and therefore a lower concentration of water) than the solution on the other side of the membrane. Water will move from the hypotonic solution into the hypertonic solution, causing the cell to lose water and potentially shrink or plasmolyze. This is why placing animal cells in a hypertonic solution can lead to cell shrinkage and death.
The Significance of Osmosis in Biological Systems
Osmosis plays a vital role in numerous biological processes across diverse organisms:
Plant Physiology: Turgor Pressure and Water Uptake
In plants, osmosis is crucial for maintaining turgor pressure, the pressure exerted by the cell contents against the cell wall. When plant cells are in a hypotonic environment, water enters the cells via osmosis, causing them to become turgid. This turgor pressure helps to maintain the plant's structure and rigidity. Wilting occurs when plants are in a hypertonic environment, leading to water loss from cells and a decrease in turgor pressure.
Animal Physiology: Maintaining Fluid Balance and Nutrient Absorption
In animals, osmosis is essential for maintaining fluid balance and nutrient absorption. The kidneys play a significant role in regulating the concentration of solutes in the blood through processes that rely heavily on osmosis. The movement of water across the membranes in the kidneys helps to regulate blood pressure and maintain a proper balance of electrolytes. Nutrient absorption in the intestines also involves osmosis, as water moves into the bloodstream to help absorb nutrients.
Cell Volume Regulation: Maintaining Homeostasis
Osmosis is fundamental in regulating cell volume. Cells constantly strive to maintain a stable internal environment, a state known as homeostasis. To achieve this, cells use various mechanisms, including osmosis, to control the movement of water and solutes across their membranes, thereby maintaining their volume and preventing damage from excessive water gain or loss.
Osmosis in Everyday Life: Practical Applications and Examples
While osmosis might seem like a purely biological phenomenon, it finds applications in various aspects of everyday life:
Food Preservation: Osmotic Drying and Salting
Osmotic drying utilizes the principle of osmosis to remove water from food products, thereby preserving them. When food is placed in a hypertonic solution (like brine), water moves out of the food, resulting in dehydration and inhibition of microbial growth. This method is used for preserving fruits, vegetables, and meats. Salting, a traditional method of food preservation, is essentially an application of osmosis, as the high salt concentration draws water out of the food, preventing bacterial growth.
Water Purification: Reverse Osmosis
Reverse osmosis is a water purification technology that utilizes pressure to overcome osmotic pressure. Water is forced through a semipermeable membrane from a region of high solute concentration to a region of low solute concentration. This process effectively removes impurities, producing clean drinking water.
Medical Applications: Dialysis
Dialysis, a life-sustaining treatment for people with kidney failure, relies on the principles of osmosis and diffusion. In dialysis, blood is passed through a semipermeable membrane that allows waste products to diffuse out while maintaining the necessary electrolytes and water balance.
Further Exploration: Advanced Concepts and Research
The study of osmosis continues to expand, with researchers exploring its role in various aspects of biology and engineering. Some areas of ongoing research include:
-
Aquaporin function and regulation: Scientists are actively studying the intricacies of aquaporin function, seeking to understand how these proteins are regulated and their precise roles in various physiological processes.
-
Osmosis in extreme environments: Research is focused on understanding how organisms survive in environments with extreme osmotic pressures, such as highly saline or arid conditions.
-
Osmosis and disease: Scientists are exploring the role of osmosis in various diseases, such as edema (fluid retention) and dehydration.
-
Applications of osmosis in biotechnology: Osmosis-based technologies are being developed for applications in drug delivery, biofuel production, and environmental remediation.
Conclusion: A Fundamental Process with Wide-Ranging Implications
Osmosis, the movement of water across a selectively permeable membrane, is a fundamental process with far-reaching implications across biology, chemistry, and even engineering. From maintaining the turgor pressure in plants to regulating fluid balance in animals and purifying water, osmosis plays a vital role in shaping life as we know it. Understanding its principles is crucial for appreciating the intricate workings of biological systems and developing innovative technologies for diverse applications. The ongoing research in this area promises to unveil further insights into the complexities of osmosis and its significance in the natural world and technological advancements.
Latest Posts
Latest Posts
-
What Is The Velocity In M Seconds Of Nerves Impules
Mar 29, 2025
-
Which Of The Following Is Not A Capital Expenditure
Mar 29, 2025
-
How Long Does It Take To Read 120 Pages
Mar 29, 2025
-
What Is An Example Of Tertiary Consumer
Mar 29, 2025
-
A Long Nonconducting Solid Cylinder Of Radius
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Osmosis Refers To The Movement Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
