The Vertical Columns On The Periodic Table Are Called
News Leon
Mar 15, 2025 · 6 min read
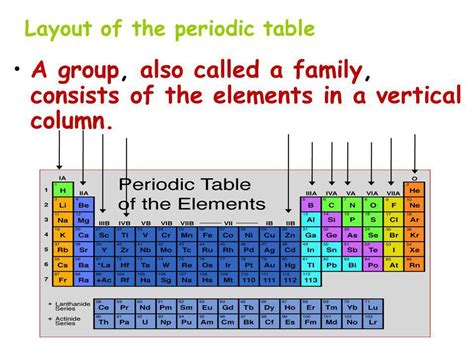
Table of Contents
The Vertical Columns on the Periodic Table are Called Groups (or Families): A Deep Dive into Chemical Properties
The periodic table, that iconic chart adorning countless science classrooms, is a testament to the organization and predictability of the chemical world. Its structure, far from arbitrary, reveals fundamental relationships between elements based on their atomic structure and resulting properties. One of the most crucial aspects of its organization lies in the vertical columns, which are formally known as groups or families. Understanding groups is fundamental to comprehending the behavior of elements and predicting their interactions. This article will delve deep into the world of periodic table groups, exploring their characteristics, trends, and significance in chemistry.
Understanding the Organization of the Periodic Table
Before diving into the specifics of groups, it's crucial to understand the overall arrangement of the periodic table. Elements are arranged in increasing order of their atomic number (the number of protons in the nucleus). This arrangement, however, isn't random. It reflects the periodic recurrence of similar chemical properties. This periodicity is due to the repeating pattern of electron configurations in the outermost electron shell, also known as the valence shell. The electrons in this shell are the primary players in chemical bonding and reactions.
The horizontal rows are called periods, and each period represents a principal energy level (shell) being filled with electrons. As you move across a period, the number of protons and electrons increases, leading to changes in atomic size and properties. However, it's the vertical columns, the groups or families, that exhibit the most striking similarities in chemical behavior.
Groups: Shared Electron Configurations and Similar Properties
Elements within the same group share a similar valence electron configuration. This means they have the same number of electrons in their outermost shell. This shared electron configuration directly impacts their chemical reactivity and the types of bonds they form. For instance, elements in Group 1, the alkali metals, all have one valence electron. This single electron readily participates in chemical reactions, making alkali metals highly reactive. Similarly, elements in Group 18, the noble gases, have a full valence shell (typically eight electrons, except for helium with two), making them incredibly unreactive.
This similarity in electron configuration translates into similar chemical and physical properties within a group. While there are variations within a group, generally, elements exhibit predictable trends in:
-
Atomic Radius: Atomic radius tends to increase as you move down a group. This is because additional electron shells are added, increasing the distance between the nucleus and the outermost electrons.
-
Ionization Energy: Ionization energy, the energy required to remove an electron from an atom, generally decreases as you move down a group. This is because the outermost electrons are further from the nucleus and therefore less strongly attracted.
-
Electronegativity: Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally decreases as you move down a group. This is due to the increasing atomic radius, which weakens the attraction between the nucleus and bonding electrons.
-
Reactivity: Reactivity is closely tied to the valence electron configuration. Groups with partially filled valence shells tend to be more reactive than those with full valence shells.
Exploring Specific Groups: A Closer Look
Let's delve deeper into several key groups to highlight the specific characteristics and properties of their members:
Group 1: Alkali Metals
The alkali metals (lithium, sodium, potassium, rubidium, cesium, and francium) are highly reactive metals. Their single valence electron is easily lost, forming +1 ions. They react vigorously with water, producing hydrogen gas and a metal hydroxide. Their reactivity increases as you move down the group due to decreasing ionization energy.
Group 2: Alkaline Earth Metals
The alkaline earth metals (beryllium, magnesium, calcium, strontium, barium, and radium) have two valence electrons, which they readily lose to form +2 ions. They are less reactive than the alkali metals but still participate in various chemical reactions. Their reactivity also increases down the group.
Group 17: Halogens
The halogens (fluorine, chlorine, bromine, iodine, and astatine) are highly reactive nonmetals. They have seven valence electrons and readily gain one electron to form -1 ions, achieving a stable octet configuration. Their reactivity decreases as you move down the group.
Group 18: Noble Gases
The noble gases (helium, neon, argon, krypton, xenon, and radon) are exceptionally unreactive. They have a full valence shell (eight electrons for most, except helium with two), making them very stable and resistant to forming chemical bonds. Their inertness has made them useful in various applications, including lighting and shielding.
Transition Metals: A Unique Group
The transition metals occupy the central block of the periodic table. They are characterized by partially filled d orbitals, leading to variable oxidation states and a wide range of complex ions. Their properties are less predictable than those in the main group elements, with a more complex interplay of electronic structure and chemical behavior.
Inner Transition Metals: Lanthanides and Actinides
The lanthanides and actinides, located at the bottom of the periodic table, represent another significant group with unique properties stemming from their partially filled f orbitals. These elements display complex chemistry, including variable oxidation states and strong tendencies to form complex ions.
The Significance of Groups in Chemistry and Beyond
Understanding the groups on the periodic table is not merely an academic exercise. It's a fundamental tool for:
-
Predicting Chemical Reactions: Knowing the group of an element allows chemists to predict its reactivity and how it will interact with other elements. This is crucial in designing chemical reactions and synthesizing new compounds.
-
Designing Materials: The properties of elements within a group influence the properties of materials they compose. Understanding group trends allows materials scientists to design materials with specific properties, such as strength, conductivity, or reactivity.
-
Understanding Biological Processes: Many biological processes depend on specific elements and their interactions. Knowing the group of an element can provide insight into its role in biological systems.
-
Technological Advancements: The properties of elements within specific groups have driven many technological advancements. For example, the unique properties of noble gases have led to their use in lighting and lasers, while the reactivity of alkali metals has been exploited in various applications.
Conclusion: Groups - The Key to Understanding Chemical Behavior
The vertical columns, or groups, on the periodic table represent a fundamental organizational principle that reflects the underlying order in the chemical world. The shared valence electron configurations within groups lead to similar chemical and physical properties, allowing for predictable trends and enabling chemists to understand and manipulate the behavior of elements. From predicting chemical reactions to designing new materials, the understanding of groups is essential for advancements in chemistry and countless related fields. The periodic table, with its carefully organized groups and periods, remains a powerful tool that continues to shape our understanding of the universe and its constituent elements. Mastering the concepts related to groups is paramount to success in the study of chemistry.
Latest Posts
Latest Posts
-
What Is The Least Common Multiple Of 4 And 9
Mar 15, 2025
-
Iodine Is Essential For The Synthesis Of
Mar 15, 2025
-
Is Osmosis High To Low Or Low To High
Mar 15, 2025
-
Concave Mirror And Convex Mirror Difference
Mar 15, 2025
-
Which Is Not A Cranial Bone Of The Skull
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about The Vertical Columns On The Periodic Table Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
