The Unit Of Energy In Si System Is
News Leon
Mar 24, 2025 · 6 min read
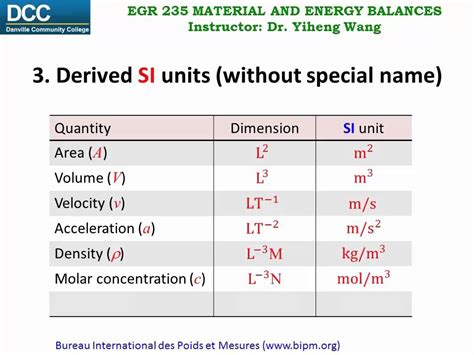
Table of Contents
- The Unit Of Energy In Si System Is
- Table of Contents
- The Unit of Energy in the SI System Is: A Deep Dive into Joules
- What is a Joule?
- Defining Work and Energy
- Different Forms of Energy Measured in Joules
- 1. Kinetic Energy
- 2. Potential Energy
- 3. Thermal Energy (Heat)
- 4. Chemical Energy
- 5. Electrical Energy
- Joules in Everyday Life and Beyond
- Conversions and Relationships with Other Energy Units
- The Significance of the Joule in Science and Engineering
- Beyond the Basics: Advanced Concepts
- Conclusion: The Joule's Enduring Legacy
- Latest Posts
- Latest Posts
- Related Post
The Unit of Energy in the SI System Is: A Deep Dive into Joules
The International System of Units (SI), the modern form of the metric system, provides a globally consistent framework for measurement. Understanding its fundamental units is crucial in various scientific and engineering fields. This article delves deep into the SI unit of energy, the joule, exploring its definition, applications, conversions, and significance in different contexts. We'll also touch upon related concepts and their connections to the joule.
What is a Joule?
The joule (J), named after the 19th-century British physicist James Prescott Joule, is the SI unit of energy. It represents the amount of work done when a force of one newton (N) is applied over a distance of one meter (m). This seemingly simple definition encapsulates a fundamental concept in physics: the ability to do work or transfer heat.
Defining Work and Energy
Work, in physics, is a measure of energy transfer that occurs when an object is moved over a distance by an external force. The formula for work is:
Work (W) = Force (F) x Distance (d) x cos θ
where θ is the angle between the force vector and the displacement vector. When the force and displacement are in the same direction, cos θ = 1, simplifying the equation to W = Fd. This aligns perfectly with the definition of a joule: 1 joule is the work done when a force of 1 newton moves an object 1 meter in the direction of the force.
Energy, conversely, represents the capacity of a system to do work. Energy exists in various forms – kinetic energy (energy of motion), potential energy (stored energy), thermal energy (heat), chemical energy, and more. The joule serves as the universal unit to quantify all these forms.
Different Forms of Energy Measured in Joules
The versatility of the joule stems from its ability to quantify diverse energy types. Let's explore some examples:
1. Kinetic Energy
Kinetic energy is the energy an object possesses due to its motion. The formula for kinetic energy is:
Kinetic Energy (KE) = 1/2 * mass (m) * velocity² (v²)
The resulting units are kg⋅m²/s², which is equivalent to a joule. A heavier object moving faster possesses greater kinetic energy, measurable in joules.
2. Potential Energy
Potential energy is stored energy that depends on the object's position or configuration. Gravitational potential energy is a common example:
Gravitational Potential Energy (PE) = mass (m) * gravitational acceleration (g) * height (h)
Again, the resulting units are kg⋅m²/s², equivalent to a joule. An object higher above the ground has greater gravitational potential energy. Other forms of potential energy, like elastic potential energy (stored in a stretched spring), are also measured in joules.
3. Thermal Energy (Heat)
Heat transfer, a form of energy transfer, is also quantified in joules. When heat energy is added to a system, it increases its internal energy, often manifesting as a temperature rise. Calorimetry experiments directly measure heat transfer in joules. The calorie, a historical unit of energy, is often converted to joules (1 calorie ≈ 4.184 joules).
4. Chemical Energy
Chemical energy, stored in the bonds of molecules, is released during chemical reactions. The energy released or absorbed in these reactions is measured in joules. For example, the energy content of food is often expressed in kilojoules (kJ), representing the chemical energy the body can extract through digestion.
5. Electrical Energy
Electrical energy, the energy carried by moving electric charges, is also measured in joules. The amount of electrical energy used by an appliance depends on its power consumption (watts) and the duration of use (seconds). The relationship is expressed as:
Energy (E) = Power (P) * Time (t)
where power is measured in watts (W) and time in seconds (s). Since 1 watt = 1 joule/second, the energy consumed is directly in joules.
Joules in Everyday Life and Beyond
While the joule might seem like a purely scientific unit, it's deeply embedded in our daily experiences:
- Food energy: Nutritional labels often display the energy content of food in kilojoules (kJ) or kilocalories (kcal), reflecting the chemical energy available for metabolic processes.
- Electricity bills: Your electricity bill reflects the total energy consumption in kilowatt-hours (kWh). One kWh is equivalent to 3.6 million joules.
- Mechanical work: Any form of mechanical work, from lifting weights to operating machinery, involves energy transfer in joules.
- Nuclear reactions: The immense energy released in nuclear reactions, such as those powering the sun, is also measured in joules, albeit on an astronomically larger scale.
Conversions and Relationships with Other Energy Units
The joule's universality is further emphasized by its relationship with other energy units:
- Electronvolt (eV): Used in atomic and nuclear physics, one eV is the energy gained by a single electron accelerating through a potential difference of one volt. It's a very small unit, with 1 eV ≈ 1.602 x 10⁻¹⁹ J.
- Calorie (cal): An older unit of energy, often used in nutrition and thermodynamics, 1 cal ≈ 4.184 J.
- Kilowatt-hour (kWh): A common unit for electricity consumption, 1 kWh = 3.6 x 10⁶ J.
- British Thermal Unit (BTU): Used primarily in the United States, 1 BTU ≈ 1055 J.
Understanding these conversions is essential for seamless transitioning between different energy scales and applications.
The Significance of the Joule in Science and Engineering
The adoption of a single, unified unit for energy has revolutionized scientific communication and engineering calculations. The joule's significance extends across numerous fields:
- Physics: It forms the cornerstone of numerous fundamental equations, simplifying energy calculations in mechanics, thermodynamics, and electromagnetism.
- Chemistry: Joules are used to quantify the energy changes in chemical reactions, crucial for understanding reaction rates and equilibrium.
- Engineering: Engineers use joules to design energy-efficient systems, analyze power transmission, and optimize performance across various applications, from mechanical systems to electrical grids.
- Environmental science: It's essential for analyzing energy flow in ecosystems, assessing the impact of energy consumption on the environment, and developing sustainable energy solutions.
Beyond the Basics: Advanced Concepts
The journey into the world of the joule goes beyond its basic definition. More advanced concepts build upon this foundation:
- Energy conservation: The principle of energy conservation states that energy cannot be created or destroyed, only transformed from one form to another. The joule provides a consistent measure to track these transformations.
- Work-energy theorem: This theorem states that the net work done on an object is equal to the change in its kinetic energy. The joule ensures consistent units in this crucial relationship.
- Power: Power, the rate at which energy is transferred or converted, is measured in watts (W), which is equivalent to joules per second (J/s).
Conclusion: The Joule's Enduring Legacy
The joule, as the SI unit of energy, represents a fundamental achievement in measurement standardization. Its widespread adoption across scientific disciplines and engineering practices has significantly simplified calculations, improved communication, and enabled advancements in various technological fields. Understanding its definition, applications, and conversions is crucial for anyone working with energy-related concepts, from students to seasoned professionals. The joule’s enduring legacy lies in its ability to unify the measurement of energy in all its diverse forms, solidifying its place as a cornerstone of the SI system.
Latest Posts
Latest Posts
-
Why Did Russia Withdraw From Ww1
Mar 26, 2025
-
Thylakoids Are Arranged In Stacks Called
Mar 26, 2025
-
Bundles Of Muscle Fibers Are Called
Mar 26, 2025
-
What Is 1 6 Of An Hour
Mar 26, 2025
-
In A Hypertonic Solution A Cell Will
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about The Unit Of Energy In Si System Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
