The Subatomic Particle With A Negative Charge Is The
News Leon
Mar 30, 2025 · 7 min read
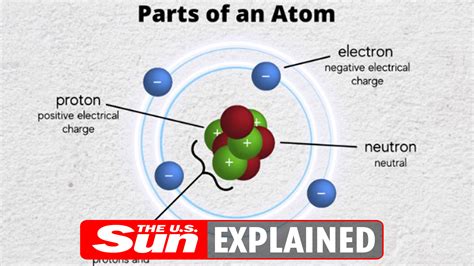
Table of Contents
The Subatomic Particle with a Negative Charge Is the Electron: A Deep Dive
The fundamental building blocks of matter, atoms, are themselves composed of even smaller particles: protons, neutrons, and electrons. While protons and neutrons reside within the atom's nucleus, electrons orbit this central core. The question, "The subatomic particle with a negative charge is the...?" has a simple answer: the electron. But the story of the electron is far more complex and fascinating than this single sentence suggests. This article will explore the electron's history, properties, behavior, and its crucial role in the universe, delving into its impact on everything from electricity to the structure of matter itself.
A Brief History of the Electron's Discovery
The discovery of the electron wasn't a single eureka moment but rather a culmination of decades of scientific investigation. Several key experiments and observations paved the way for its identification:
Cathode Rays and J.J. Thomson's Experiment
In the late 19th century, scientists were experimenting with cathode ray tubes – sealed glass tubes with electrodes at each end. When a high voltage was applied, rays emanated from the cathode (negative electrode) and traveled to the anode (positive electrode). These rays, known as cathode rays, exhibited several peculiar properties:
- Deflection by magnetic fields: The rays were deflected by magnetic fields, suggesting they carried an electric charge.
- Independent of cathode material: The properties of the rays remained consistent regardless of the material used for the cathode, indicating a fundamental constituent of matter.
It was J.J. Thomson who, in 1897, conducted a series of experiments that definitively showed cathode rays were streams of negatively charged particles, far smaller than any previously known atom. He determined their charge-to-mass ratio, a crucial step in identifying them as a new subatomic particle – the electron. This groundbreaking work revolutionized our understanding of the atom, shattering the prevailing belief that atoms were indivisible.
Millikan's Oil Drop Experiment
While Thomson determined the charge-to-mass ratio, the exact charge and mass of the electron remained unknown. Robert Millikan's oil drop experiment, conducted in 1909, precisely measured the electron's charge. By observing the motion of tiny oil droplets suspended in an electric field, Millikan meticulously determined the fundamental unit of electric charge, which corresponded to the charge of a single electron. This, combined with Thomson's work, allowed for the calculation of the electron's mass.
Properties of the Electron
The electron possesses several key properties that define its behavior and role in the universe:
Charge
The electron carries a fundamental unit of negative electric charge, conventionally denoted as -1. This negative charge is exactly equal in magnitude to the positive charge of a proton. This balance of charges is crucial for the stability of atoms and molecules.
Mass
The electron's mass is incredibly small, approximately 9.109 × 10<sup>-31</sup> kilograms. This is significantly less than the mass of a proton or neutron, which are roughly 1836 and 1839 times more massive, respectively.
Spin
Electrons possess an intrinsic angular momentum called spin. This isn't a literal spinning motion, but rather a quantum mechanical property that manifests as an intrinsic magnetic moment. The spin of an electron can be either "up" or "down," which plays a crucial role in determining the chemical properties of atoms.
Wave-Particle Duality
One of the most remarkable aspects of the electron is its wave-particle duality. This means that the electron exhibits properties of both a particle (possessing mass and charge) and a wave (exhibiting wave-like behavior such as diffraction and interference). This duality is a cornerstone of quantum mechanics and highlights the limitations of classical physics in describing the behavior of subatomic particles.
The Electron's Role in Atoms and Molecules
Electrons are pivotal in determining the properties of atoms and molecules:
Atomic Structure
Electrons orbit the atom's nucleus, which contains protons and neutrons. The number of electrons in an atom determines its chemical properties and how it will interact with other atoms. Electrons occupy specific energy levels or shells around the nucleus, and the arrangement of these electrons defines the atom's electronic configuration.
Chemical Bonding
Electrons are directly involved in chemical bonding, the forces that hold atoms together to form molecules. There are several types of chemical bonds:
- Ionic bonding: Involves the transfer of electrons from one atom to another, creating ions with opposite charges that attract each other.
- Covalent bonding: Involves the sharing of electrons between atoms, creating a stable molecule.
- Metallic bonding: Involves a "sea" of delocalized electrons shared among a lattice of metal atoms.
The behavior of electrons during chemical bonding dictates the properties of the resulting molecules, such as their reactivity, strength, and other physical characteristics.
Electrical Conductivity
Electrons are the primary carriers of electric current in materials. In conductors, such as metals, electrons are relatively free to move throughout the material, allowing for the easy flow of electricity. In insulators, electrons are tightly bound to their atoms and cannot move freely, preventing the flow of electricity. Semiconductors fall between these two extremes, exhibiting controllable electrical conductivity.
The Electron in Technology and Beyond
The electron's properties and behavior have been harnessed in a wide range of technologies:
Electronics
The vast majority of modern electronic devices rely on the controlled flow of electrons. Transistors, integrated circuits, and other electronic components manipulate electron flow to process information and perform computations. Without the electron, our modern digital world would be impossible.
Imaging Techniques
Electrons play a significant role in various imaging techniques, including:
- Electron microscopy: Uses a beam of electrons to create highly magnified images of materials, revealing structures far smaller than those visible with light microscopes.
- X-ray technology: While X-rays are electromagnetic radiation, their generation often involves the interaction of electrons with atoms.
Particle Accelerators
Particle accelerators, such as the Large Hadron Collider, use powerful electric and magnetic fields to accelerate electrons (and other particles) to incredibly high speeds, allowing scientists to study the fundamental forces of nature and the structure of matter at its most basic level.
Beyond the Basics: Deeper Explorations of Electron Behavior
The electron's behavior is governed by the laws of quantum mechanics, a field of physics that deals with the bizarre and counterintuitive world of the subatomic. Several advanced concepts help to explain the electron’s intricate behavior:
Quantum Numbers
Electrons in atoms are described by a set of four quantum numbers:
- Principal quantum number (n): Determines the energy level of the electron.
- Azimuthal quantum number (l): Determines the shape of the electron's orbital.
- Magnetic quantum number (ml): Determines the orientation of the electron's orbital in space.
- Spin quantum number (ms): Determines the electron's spin.
These quantum numbers uniquely identify each electron in an atom and are essential for understanding atomic structure and chemical bonding.
Pauli Exclusion Principle
The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers. This principle dictates how electrons fill the energy levels of an atom and is essential for understanding the periodic table of elements and the chemical properties of different elements.
Heisenberg Uncertainty Principle
The Heisenberg Uncertainty Principle states that it is impossible to simultaneously know both the position and momentum of an electron with perfect accuracy. This principle reflects the inherent uncertainty in the behavior of subatomic particles and highlights the limitations of classical physics in describing their behavior.
Electron Configuration and the Periodic Table
The arrangement of electrons in an atom's energy levels, known as its electron configuration, determines its chemical properties. The periodic table is organized according to electron configurations, reflecting the recurring patterns in the chemical properties of elements.
The Electron's Ongoing Significance in Scientific Research
The electron continues to be a central focus of scientific research. Ongoing research explores various aspects of electron behavior and its role in various phenomena:
- High-energy physics: Studies the behavior of electrons at extremely high energies, revealing insights into the fundamental forces of nature.
- Condensed matter physics: Investigates the collective behavior of electrons in solids, leading to advancements in materials science and electronics.
- Quantum computing: Explores the potential of electrons (and other quantum systems) for building powerful quantum computers that can solve problems beyond the capabilities of classical computers.
The electron, seemingly a simple negatively charged particle, is far more complex and fascinating than it initially appears. Its discovery revolutionized our understanding of matter and continues to drive cutting-edge research in physics and technology. From the fundamental building blocks of atoms to the sophisticated technologies that shape our modern world, the electron's influence is profound and pervasive. Its continued study holds the key to unlocking even deeper secrets of the universe.
Latest Posts
Latest Posts
-
Smallest Part Of An Element Or Compound
Apr 01, 2025
-
Excess Glucose In Animals Is Stored As
Apr 01, 2025
-
A Decrease In Demand Refers To
Apr 01, 2025
-
Which Enzyme Is Not Involved In Dna Replication
Apr 01, 2025
-
A Person Makes A Quantity Of Iced Tea By Mixing
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about The Subatomic Particle With A Negative Charge Is The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
