The Nucleus Of An Atom Consists Mainly Of
News Leon
Mar 26, 2025 · 6 min read
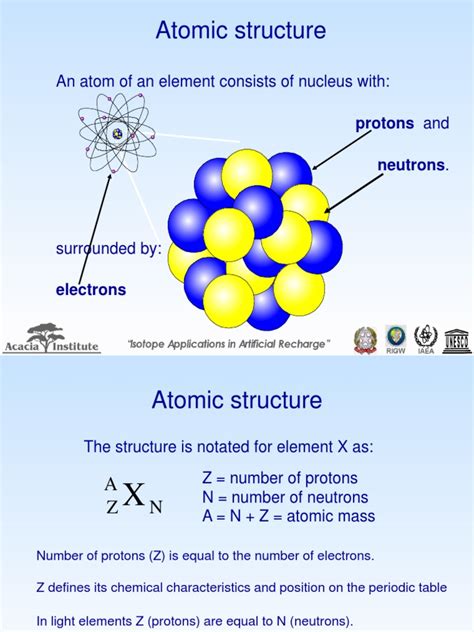
Table of Contents
The Nucleus of an Atom: A Deep Dive into Protons and Neutrons
The atom, the fundamental building block of matter, is a fascinating world of subatomic particles. While the atom as a whole is mostly empty space, its heart, the nucleus, holds the key to an element's identity and properties. This dense, central region consists primarily of two types of particles: protons and neutrons. Understanding their characteristics, interactions, and roles is crucial to comprehending the nature of matter itself. This article will delve into the intricacies of the atomic nucleus, exploring its composition, properties, and significance in various scientific fields.
The Composition of the Atomic Nucleus: Protons and Neutrons
The atomic nucleus is a tightly packed sphere of positively charged protons and electrically neutral neutrons. These particles, collectively known as nucleons, are held together by a powerful force known as the strong nuclear force. This force overcomes the electrostatic repulsion between the positively charged protons, preventing the nucleus from flying apart.
Protons: The Defining Characteristic of an Element
Protons, symbolized as 'p' or 'p⁺', carry a single positive elementary charge (+1e), where 'e' represents the elementary charge. The number of protons in an atom's nucleus defines its atomic number, a fundamental property that distinguishes one element from another. For instance, hydrogen (H) has one proton (atomic number 1), helium (He) has two protons (atomic number 2), and uranium (U) has 92 protons (atomic number 92). This number is crucial because it determines the element's position on the periodic table and dictates its chemical behavior. Altering the number of protons fundamentally changes the element itself.
Key properties of protons:
- Positive charge: Responsible for the overall positive charge of the nucleus.
- Mass: Approximately 1.6726 × 10⁻²⁷ kg, slightly less than the mass of a neutron.
- Spin: An intrinsic angular momentum of ½, contributing to the overall nuclear spin.
- Stability: Protons are remarkably stable particles, not subject to decay under normal conditions.
Neutrons: The Nuclear Glue
Neutrons, symbolized as 'n' or 'n⁰', have no net electric charge (0e). Their presence in the nucleus is essential for the stability of most atoms. While protons repel each other due to their like charges, neutrons help to overcome this repulsion through the strong nuclear force. They act as a kind of "nuclear glue," binding the protons together and stabilizing the nucleus.
The number of neutrons in an atom's nucleus is its neutron number. Atoms of the same element can have different neutron numbers, resulting in isotopes. Isotopes are atoms with the same number of protons but different numbers of neutrons. Some isotopes are stable, while others are radioactive, meaning they decay over time, emitting radiation.
Key properties of neutrons:
- Neutral charge: Contributes to the overall neutrality of the nucleus when balanced with protons.
- Mass: Approximately 1.6749 × 10⁻²⁷ kg, slightly greater than the mass of a proton.
- Spin: An intrinsic angular momentum of ½, interacting with proton spins to determine the overall nuclear spin.
- Stability: Free neutrons are unstable and decay into protons, electrons, and antineutrinos with a half-life of about 10 minutes. However, within the nucleus, neutrons can be stable, contributing to the overall nuclear stability.
The Strong Nuclear Force: The Force that Holds the Nucleus Together
The strong nuclear force is one of the four fundamental forces in nature, alongside the electromagnetic, weak nuclear, and gravitational forces. It is the strongest of these forces, but it acts only over very short distances—roughly the size of the atomic nucleus. Its strength is crucial in overcoming the electrostatic repulsion between protons, thereby holding the nucleus together.
The strong force is not simply an attractive force between nucleons; its complexity stems from its dependence on several factors:
- Distance: The force is strongest at very short distances and rapidly weakens as the distance between nucleons increases. This explains why nuclei are so compact.
- Isospin: Nucleons exhibit a property called isospin, which acts like a kind of "nuclear charge." The strong force is equally strong between protons and neutrons, exhibiting a type of isospin symmetry.
- Quantum Chromodynamics (QCD): Our modern understanding of the strong force comes from QCD, a branch of physics that describes the force in terms of the interactions between quarks and gluons, the fundamental constituents of protons and neutrons.
Isotopes and Nuclear Stability
As mentioned earlier, isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. The stability of an isotope is determined by the balance between the strong nuclear force and the electrostatic repulsion between protons.
- Stable Isotopes: These isotopes have a stable neutron-to-proton ratio, allowing the strong force to effectively overcome the electrostatic repulsion. The majority of elements have one or more stable isotopes.
- Unstable (Radioactive) Isotopes: These isotopes have an unstable neutron-to-proton ratio, resulting in an imbalance between the strong force and electrostatic repulsion. This imbalance leads to radioactive decay, where the nucleus emits particles or energy to reach a more stable configuration. Radioactive isotopes have various applications in medicine, research, and industrial processes.
Nuclear Reactions: Fission and Fusion
The nucleus can undergo various transformations, known as nuclear reactions. Two significant types of nuclear reactions are:
- Nuclear Fission: The splitting of a heavy atomic nucleus into two or more lighter nuclei. This process releases a tremendous amount of energy, as evidenced by nuclear power plants and atomic bombs. Fission occurs when a heavy nucleus absorbs a neutron, destabilizing it and causing it to split.
- Nuclear Fusion: The combining of two lighter atomic nuclei to form a heavier nucleus. This process also releases a significant amount of energy, powering the sun and other stars. Fusion requires extremely high temperatures and pressures to overcome the electrostatic repulsion between the nuclei.
The Significance of the Atomic Nucleus
The atomic nucleus plays a critical role in various fields of science and technology:
- Nuclear Medicine: Radioactive isotopes are used in diagnostic imaging techniques like PET (positron emission tomography) and SPECT (single-photon emission computed tomography) scans, as well as in radiation therapy for cancer treatment.
- Nuclear Energy: Nuclear fission is used in nuclear power plants to generate electricity.
- Materials Science: Understanding nuclear properties is crucial for developing new materials with specific characteristics, such as strength, conductivity, and reactivity.
- Archaeology and Geology: Radioactive dating techniques, utilizing the decay of radioactive isotopes, are used to determine the age of artifacts and geological formations.
Conclusion
The atomic nucleus, a tiny but incredibly powerful entity, is composed primarily of protons and neutrons held together by the strong nuclear force. The number of protons defines the element, while the number of neutrons influences its stability and isotopic properties. Understanding the structure and behavior of the atomic nucleus is fundamental to numerous scientific disciplines and technological advancements. From nuclear energy to medical imaging, the significance of the nucleus is undeniable, making its continued study vital for future discoveries and applications. Further research into the intricacies of the strong nuclear force and the behavior of nucleons promises to unlock even deeper insights into the fundamental nature of matter and the universe itself.
Latest Posts
Latest Posts
-
Saturation Water Vapor Pressure Increases With Temperature
Mar 29, 2025
-
The Structural Unit Of Compact Bone Is The
Mar 29, 2025
-
Complete And Balance The The Following Equation
Mar 29, 2025
-
An Electron That Has A Velocity With X Component
Mar 29, 2025
-
A Food Chain Always Starts With
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about The Nucleus Of An Atom Consists Mainly Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
