The Mass Per Unit Volume Of A Substance
News Leon
Mar 16, 2025 · 7 min read
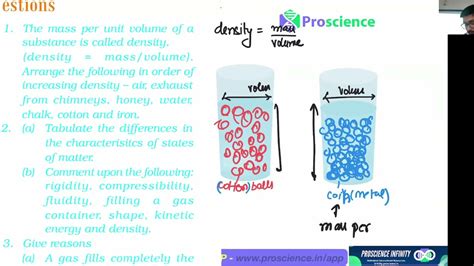
Table of Contents
The Mass Per Unit Volume of a Substance: Density Explained
Density, the mass per unit volume of a substance, is a fundamental concept in physics and chemistry with far-reaching applications across numerous scientific disciplines and everyday life. Understanding density allows us to predict the behavior of materials, design efficient structures, and solve a wide array of problems. This comprehensive guide delves into the intricacies of density, exploring its definition, calculation, units, significance, and practical applications.
Defining Density: Mass Packed into Space
Density (ρ, pronounced "rho") is a scalar quantity that describes how much mass is packed into a given volume. It's essentially a measure of how tightly the atoms or molecules of a substance are packed together. A high-density material has a large mass concentrated in a small volume, while a low-density material has a smaller mass spread over a larger volume. The formal definition is:
Density = Mass / Volume
This simple equation forms the cornerstone of all density calculations. It emphasizes the direct proportionality between mass and density (higher mass means higher density, assuming constant volume) and the inverse proportionality between volume and density (higher volume means lower density, assuming constant mass).
Units of Density: A Matter of Scale
The units of density are derived directly from its defining equation: mass divided by volume. The most common units include:
-
kg/m³ (kilograms per cubic meter): This is the SI (International System of Units) unit for density and is widely used in scientific and engineering applications.
-
g/cm³ (grams per cubic centimeter): This unit is particularly convenient for measuring the density of solids and liquids, as it often yields numbers within a manageable range. Note that 1 g/cm³ is exactly equal to 1000 kg/m³.
-
g/mL (grams per milliliter): This unit is equivalent to g/cm³ and is frequently used when dealing with liquids.
-
lb/ft³ (pounds per cubic foot): This unit is commonly used in engineering applications in the United States.
Calculating Density: Methods and Considerations
Calculating the density of a substance involves determining both its mass and its volume. The methods employed depend on the state of the substance (solid, liquid, or gas) and the available equipment.
Measuring Mass: A Weighing Matter
Mass is typically measured using a balance or scale. For precise measurements, analytical balances are used, capable of measuring masses to several decimal places. The accuracy of the mass measurement is crucial for obtaining an accurate density value.
Measuring Volume: Different Approaches for Different States
Measuring volume depends on the state of the matter:
-
Solids: For regularly shaped solids (cubes, spheres, cylinders), the volume can be calculated using geometric formulas. For irregularly shaped solids, the volume can be determined using water displacement: Submerge the solid in a known volume of water and measure the increase in water level. The increase in volume is equal to the volume of the solid.
-
Liquids: The volume of liquids can be measured using graduated cylinders, pipettes, burets, or volumetric flasks, depending on the desired precision.
-
Gases: Measuring the volume of gases requires specialized equipment, such as gas burettes or calibrated containers, and consideration of temperature and pressure, as gas volume is highly sensitive to these parameters.
Density Calculation: Putting it All Together
Once the mass and volume have been determined, the density can be calculated using the formula:
Density = Mass / Volume
For example, if a 100g block of wood occupies a volume of 125 cm³, its density is:
Density = 100g / 125 cm³ = 0.8 g/cm³
Factors Affecting Density: Temperature, Pressure, and Composition
Several factors can influence the density of a substance:
-
Temperature: Temperature affects the density of most substances. Generally, an increase in temperature leads to an increase in volume and, therefore, a decrease in density (for exceptions like water near its freezing point, see below). This is because higher temperatures cause molecules to move faster and occupy more space.
-
Pressure: Pressure primarily affects the density of gases. Increasing the pressure on a gas reduces its volume, thereby increasing its density. The effect of pressure on the density of liquids and solids is generally negligible.
-
Composition: The chemical composition of a substance directly affects its density. Different substances have different atomic or molecular structures and masses, leading to variations in density. For mixtures and solutions, the density is dependent on the proportions of the components.
-
Phase: The phase of a substance (solid, liquid, or gas) significantly impacts its density. Generally, solids are denser than liquids, and liquids are denser than gases. This is due to the differences in intermolecular forces and the arrangement of particles in each phase.
Density Anomalies: Water's Unique Behavior
Water exhibits a unique density anomaly. Unlike most substances, the density of water is highest at 4°C (39.2°F). As water cools below 4°C, its density decreases, and ice (solid water) is less dense than liquid water at 0°C. This unusual behavior is due to the hydrogen bonding in water molecules, which leads to a unique arrangement in the solid state. This anomaly is crucial for aquatic life, as it prevents bodies of water from freezing solid from the bottom up.
Applications of Density: A Wide Range of Uses
Density measurements find applications in numerous fields:
-
Material Identification: Density is a characteristic property of a substance, allowing for its identification. Comparing the measured density of an unknown sample to known values can help determine its composition.
-
Gemology: The density of gemstones is an important parameter for identifying and authenticating precious stones. Density testing is a crucial tool in gemological analysis.
-
Medicine: Density measurements are used in various medical applications, including bone density scans (osteoporosis diagnosis) and blood tests (measuring hematocrit, the proportion of red blood cells in blood).
-
Industrial Processes: Density measurements are crucial in various industrial processes, such as controlling the concentration of solutions, monitoring the quality of products, and ensuring efficient mixing of materials.
-
Oceanography: Oceanographers measure water density to understand ocean currents, salinity variations, and temperature profiles. These measurements are crucial for understanding marine ecosystems and climate change.
-
Aerospace Engineering: Density plays a significant role in aerospace engineering, influencing aircraft design, fuel efficiency, and atmospheric flight conditions.
Density and Buoyancy: Floating and Sinking
Density is intrinsically linked to buoyancy – the upward force exerted on an object submerged in a fluid. Archimedes' principle states that the buoyant force on an object is equal to the weight of the fluid displaced by the object. If the density of the object is less than the density of the fluid, the buoyant force is greater than the weight of the object, and it floats. If the density of the object is greater than the density of the fluid, the buoyant force is less than the weight of the object, and it sinks. This principle is essential in understanding ship design, submarine operation, and the behavior of objects in different fluids.
Advanced Concepts: Relative Density and Specific Gravity
While density itself is a crucial concept, related terms further enhance our understanding:
-
Relative Density: Relative density (or specific gravity) is the ratio of the density of a substance to the density of a reference substance, typically water at 4°C. It is a dimensionless quantity, providing a convenient way to compare the densities of different materials. For example, a substance with a relative density of 2 is twice as dense as water.
-
Apparent Density: Apparent density refers to the density of a material considering its pores or voids. It's lower than the true density, which considers the material's solid structure alone. This distinction is crucial when working with porous materials like soil or rocks.
Conclusion: Density – A Cornerstone of Understanding the Physical World
Density, the mass per unit volume, is a fundamental concept with widespread implications across science, engineering, and everyday life. Understanding its definition, calculation, and influencing factors is essential for tackling various scientific and practical problems. From identifying materials to understanding buoyancy and designing efficient structures, the significance of density cannot be overstated. This comprehensive exploration of density serves as a valuable resource for students, researchers, and anyone seeking a deeper understanding of this critical physical property.
Latest Posts
Latest Posts
-
How Many Chromosomes In The Human Egg Cell
Mar 16, 2025
-
What Has A Face And Two Hands
Mar 16, 2025
-
What Is The Difference Between Ml And Mg
Mar 16, 2025
-
Having Two Different Alleles For A Trait
Mar 16, 2025
-
To What Does The Term Grana Refer
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about The Mass Per Unit Volume Of A Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
