The Mass Number Of An Atom Is Determined By
News Leon
Mar 23, 2025 · 5 min read
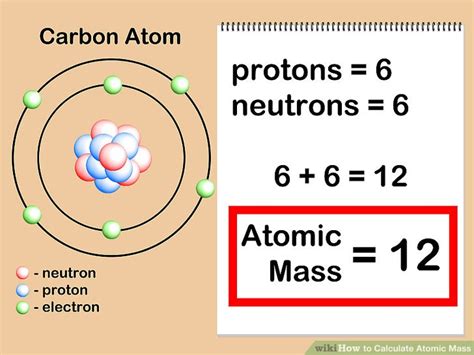
Table of Contents
The Mass Number of an Atom: A Deep Dive into Atomic Structure and Isotopes
Understanding the mass number of an atom is fundamental to grasping the basics of chemistry and nuclear physics. This seemingly simple number holds a wealth of information about an atom's composition and properties, influencing its behavior in chemical reactions and nuclear processes. This article will explore the intricacies of atomic mass, its relationship to protons and neutrons, the concept of isotopes, and the implications of mass number in various scientific fields.
What is Mass Number?
The mass number (A) of an atom is the total number of protons and neutrons found in its nucleus. It's a whole number, representing the approximate mass of the atom in atomic mass units (amu). Crucially, it doesn't include the mass of electrons, as electrons are significantly lighter than protons and neutrons and contribute negligibly to the overall mass.
In simple terms: Mass number = Number of protons + Number of neutrons
This formula is a cornerstone of understanding atomic structure. Let's delve deeper into the individual components:
Protons: The Defining Characteristic
Protons, positively charged particles residing within the atom's nucleus, determine the atom's atomic number (Z). The atomic number uniquely identifies an element; all atoms of a given element have the same number of protons. For example, all hydrogen atoms have one proton (Z=1), all helium atoms have two protons (Z=2), and so on. The atomic number is crucial in organizing the periodic table, reflecting the periodic trends in elemental properties.
Neutrons: The Mass Contributors
Neutrons, neutrally charged particles also located in the nucleus, contribute significantly to an atom's mass but not to its chemical identity. Isotopes, which we will discuss in detail below, are atoms of the same element (same number of protons) but with differing numbers of neutrons, resulting in variations in mass number.
Isotopes: Variations on a Theme
Isotopes are atoms of the same element that possess the same number of protons but a different number of neutrons. This difference in neutron number leads to variations in their mass number. For instance, carbon-12 (¹²C), carbon-13 (¹³C), and carbon-14 (¹⁴C) are all isotopes of carbon. They all have six protons (Z=6), but their neutron numbers are 6, 7, and 8 respectively, resulting in mass numbers of 12, 13, and 14.
Understanding Isotopic Notation:
Isotopes are represented using isotopic notation: ^A_Z X, where:
- A is the mass number (protons + neutrons)
- Z is the atomic number (number of protons)
- X is the element symbol
For example, ^14_6C represents carbon-14, with a mass number of 14 and an atomic number of 6.
Abundance and Average Atomic Mass
Isotopes exist naturally in varying abundances. The average atomic mass of an element, as listed on the periodic table, is a weighted average of the masses of its isotopes, considering their relative abundances. This weighted average reflects the typical mass of an atom of that element found in nature.
For example, chlorine has two main isotopes: chlorine-35 (⁷⁵% abundance) and chlorine-37 (²⁵% abundance). The average atomic mass of chlorine is calculated as follows:
(0.75 * 35 amu) + (0.25 * 37 amu) = 35.5 amu
This average atomic mass is essential in stoichiometric calculations and other chemical analyses.
The Significance of Mass Number in Various Scientific Fields
The mass number is a critical parameter in various scientific fields:
Nuclear Chemistry and Physics:
- Nuclear Reactions: Mass number is conserved in most nuclear reactions. This principle, alongside charge conservation, is used to balance nuclear equations and predict the products of nuclear reactions like fission and fusion.
- Radioactive Decay: The mass number changes during certain types of radioactive decay, such as alpha decay (loss of an alpha particle, ⁴₂He), providing insights into the decay process and the resulting daughter isotopes.
- Nuclear Stability: The ratio of neutrons to protons within the nucleus influences an atom's nuclear stability. Isotopes with specific neutron-to-proton ratios are more stable than others, influencing the half-life and decay modes of radioactive isotopes.
- Nuclear Medicine: Radioactive isotopes with specific mass numbers are used in medical imaging and therapy. Their decay characteristics and targeted delivery to specific tissues allow for accurate diagnostics and treatments.
Chemistry:
- Isotopic Tracers: Isotopes with differing mass numbers can be used as tracers in chemical reactions and metabolic processes. By tracking the movement of these labeled atoms, scientists can gain insights into reaction mechanisms and biological pathways.
- Mass Spectrometry: Mass spectrometry is a powerful analytical technique that separates ions based on their mass-to-charge ratio. This technique is frequently used to identify and quantify isotopes within a sample, providing information about isotopic abundances and elemental composition.
Geology and Archaeology:
- Radiometric Dating: Radioactive isotopes with known decay rates (half-lives) are used for radiometric dating of geological samples and artifacts. The ratio of parent isotope to daughter isotope allows scientists to determine the age of materials, providing insights into Earth's history and past civilizations.
Beyond the Basics: Nuclear Binding Energy and Mass Defect
The mass number is intimately related to a fundamental concept in nuclear physics: nuclear binding energy. When protons and neutrons combine to form a nucleus, a small amount of mass is converted into a tremendous amount of energy, according to Einstein's famous equation, E=mc². This mass difference between the sum of the individual masses of protons and neutrons and the actual mass of the nucleus is known as the mass defect. The energy equivalent of this mass defect is the nuclear binding energy, which holds the nucleus together. A higher binding energy per nucleon generally indicates greater nuclear stability.
Conclusion: The Mass Number - A Key to Understanding Matter
The mass number, a seemingly simple numerical representation, is a powerful tool for understanding the structure, behavior, and properties of atoms and their isotopes. Its role extends across numerous scientific disciplines, playing a pivotal role in nuclear physics, chemistry, geology, and archaeology. By understanding the mass number's relationship to protons, neutrons, and isotopes, we gain valuable insights into the fundamental nature of matter and its transformations. The concepts discussed here provide a solid foundation for further exploration of advanced topics in atomic structure and nuclear science. From the simplest atoms to the most complex nuclear reactions, the mass number remains a cornerstone of our understanding of the physical world.
Latest Posts
Latest Posts
-
Which Of The Following Would Not Be A Physical Change
Mar 24, 2025
-
Sample 500 Words Essay About Myself
Mar 24, 2025
-
Which Of The Following Is Vector Quantity
Mar 24, 2025
-
Gof 1 F 1 Og 1 Proof
Mar 24, 2025
-
Identify The Functional Groups In The Following Compounds
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about The Mass Number Of An Atom Is Determined By . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
