Identify The Functional Groups In The Following Compounds
News Leon
Mar 24, 2025 · 8 min read
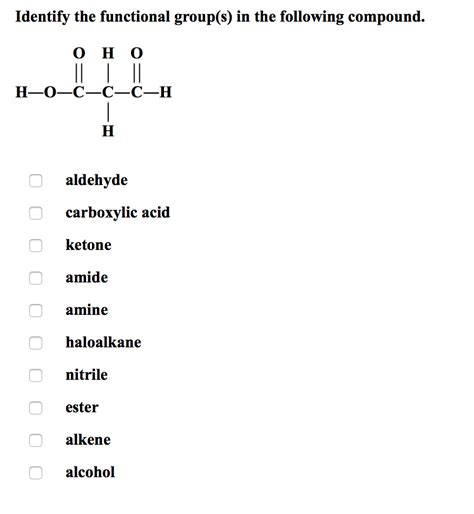
Table of Contents
Identifying Functional Groups in Organic Compounds: A Comprehensive Guide
Organic chemistry can seem daunting at first, but understanding the fundamental building blocks—functional groups—is key to mastering it. This comprehensive guide will walk you through identifying various functional groups in different organic compounds, providing clear explanations and examples. We'll explore the properties associated with each functional group, helping you develop a strong understanding of organic molecule structure and reactivity.
What are Functional Groups?
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. They are the reactive centers of organic compounds, dictating how a molecule will behave in different chemical environments. Recognizing functional groups is crucial for predicting the properties and reactivity of organic molecules.
Common Functional Groups and Their Identification
Let's delve into some of the most common functional groups, exploring their structures, properties, and how to identify them in a given compound:
1. Alkanes (C-C and C-H): The Foundation
Alkanes are the simplest organic compounds, consisting solely of carbon-carbon single bonds and carbon-hydrogen bonds. They are saturated hydrocarbons, meaning they contain only single bonds and no double or triple bonds. Alkanes are relatively unreactive compared to other functional groups.
- Structure: A chain of carbon atoms, each bonded to enough hydrogen atoms to satisfy the carbon's four valencies.
- Example: Methane (CH₄), Ethane (C₂H₆), Propane (C₃H₈)
- Identification: The presence of only single bonds between carbon atoms and hydrogen atoms. The general formula is C<sub>n</sub>H<sub>2n+2</sub> for straight-chain alkanes.
2. Alkenes (C=C): The Double Bond
Alkenes contain at least one carbon-carbon double bond (C=C). This double bond introduces unsaturation, significantly increasing the molecule's reactivity. Alkenes readily undergo addition reactions.
- Structure: One or more carbon-carbon double bonds.
- Example: Ethene (C₂H₄), Propene (C₃H₆)
- Identification: The presence of a carbon-carbon double bond. The general formula for simple alkenes is C<sub>n</sub>H<sub>2n</sub>.
3. Alkynes (C≡C): The Triple Bond
Alkynes possess at least one carbon-carbon triple bond (C≡C). This triple bond further increases reactivity compared to alkenes. Alkynes also undergo addition reactions.
- Structure: One or more carbon-carbon triple bonds.
- Example: Ethyne (C₂H₂), Propyne (C₃H₄)
- Identification: The presence of a carbon-carbon triple bond. The general formula for simple alkynes is C<sub>n</sub>H<sub>2n-2</sub>.
4. Alcohols (-OH): The Hydroxyl Group
Alcohols contain a hydroxyl group (-OH) bonded to a saturated carbon atom. The hydroxyl group's presence significantly impacts the molecule's polarity and solubility in water.
- Structure: -OH group attached to a carbon atom.
- Example: Methanol (CH₃OH), Ethanol (CH₃CH₂OH)
- Identification: The presence of an -OH group bonded to a carbon atom (not to an aromatic ring or a carbonyl carbon).
5. Ethers (-O-): Oxygen in the Middle
Ethers have an oxygen atom bonded to two carbon atoms (R-O-R'). They are relatively unreactive compared to alcohols.
- Structure: -O- group connecting two carbon atoms or alkyl groups.
- Example: Dimethyl ether (CH₃OCH₃), Diethyl ether (CH₃CH₂OCH₂CH₃)
- Identification: An oxygen atom bonded to two carbon atoms.
6. Aldehydes (-CHO): The Carbonyl at the End
Aldehydes feature a carbonyl group (C=O) at the end of a carbon chain. The carbonyl carbon is bonded to at least one hydrogen atom. Aldehydes are easily oxidized.
- Structure: -CHO group (carbonyl group at the end of the chain).
- Example: Formaldehyde (HCHO), Acetaldehyde (CH₃CHO)
- Identification: A carbonyl group (C=O) at the terminal position of a carbon chain.
7. Ketones (R-CO-R'): The Carbonyl in the Middle
Ketones also have a carbonyl group (C=O), but it's located within the carbon chain, not at the end. Ketones are less reactive than aldehydes.
- Structure: -CO- group (carbonyl group within the chain).
- Example: Acetone (CH₃COCH₃), Propanone (CH₃COCH₃)
- Identification: A carbonyl group (C=O) bonded to two carbon atoms.
8. Carboxylic Acids (-COOH): The Acidic Group
Carboxylic acids are characterized by a carboxyl group (-COOH), which is a combination of a carbonyl group and a hydroxyl group. They are acidic, readily donating a proton (H⁺).
- Structure: -COOH group (carboxyl group).
- Example: Acetic acid (CH₃COOH), Formic acid (HCOOH)
- Identification: The presence of a -COOH group.
9. Esters (-COO-): The Fruity Smell
Esters are derived from carboxylic acids and alcohols. They possess a -COO- group and often have pleasant, fruity odors.
- Structure: -COO- group (ester group).
- Example: Ethyl acetate (CH₃COOCH₂CH₃), Methyl acetate (CH₃COOCH₃)
- Identification: The -COO- group, which is derived from the reaction between a carboxylic acid and an alcohol.
10. Amines (-NH₂ , -NHR, -NR₂): Nitrogen Containing
Amines contain a nitrogen atom bonded to one, two, or three carbon atoms (or alkyl groups). They are basic, accepting protons (H⁺).
- Structure: -NH₂, -NHR, -NR₂ (amine group).
- Example: Methylamine (CH₃NH₂), Dimethylamine ((CH₃)₂NH), Trimethylamine ((CH₃)₃N)
- Identification: The presence of a nitrogen atom bonded to carbon atoms or alkyl groups.
11. Amides (-CONH₂): Nitrogen Bonded to a Carbonyl
Amides have a carbonyl group (C=O) bonded to a nitrogen atom. They are neutral, but the nitrogen can participate in hydrogen bonding.
- Structure: -CONH₂ group (amide group).
- Example: Acetamide (CH₃CONH₂), Formamide (HCONH₂)
- Identification: The -CONH₂ group, featuring both a carbonyl and a nitrogen atom.
12. Nitriles (-CN): The Cyano Group
Nitriles contain a cyano group (-CN), which consists of a carbon atom triple-bonded to a nitrogen atom.
- Structure: -CN group (cyano group).
- Example: Acetonitrile (CH₃CN)
- Identification: The presence of a -CN group (a carbon-nitrogen triple bond).
13. Aromatic Compounds (Benzene Ring): Special Stability
Aromatic compounds, such as benzene, contain a special type of ring structure with delocalized pi electrons, conferring unique stability.
- Structure: Benzene ring (a six-membered ring with alternating single and double bonds).
- Example: Benzene (C₆H₆), Toluene (C₇H₈)
- Identification: The presence of a benzene ring or related aromatic structure. Look for a six-membered ring with alternating single and double bonds, represented often as a circle within the hexagon.
14. Halogenated Compounds (-F, -Cl, -Br, -I): Halogens Attached
Halogenated compounds contain at least one halogen atom (fluorine, chlorine, bromine, or iodine) bonded to a carbon atom. These compounds often exhibit increased reactivity.
- Structure: -F, -Cl, -Br, -I (halogen atom attached to a carbon).
- Example: Chloromethane (CH₃Cl), Chloroform (CHCl₃)
- Identification: Presence of a fluorine, chlorine, bromine, or iodine atom bonded to a carbon atom.
Practice Identifying Functional Groups
To solidify your understanding, let's practice identifying functional groups in some example compounds:
Example 1: CH₃CH₂CH₂OH
This compound contains a hydroxyl group (-OH) attached to a saturated carbon, making it an alcohol.
Example 2: CH₃CH₂CHO
This compound features a carbonyl group (C=O) at the end of a carbon chain, identifying it as an aldehyde.
Example 3: CH₃COCH₃
This compound has a carbonyl group (C=O) within the carbon chain, making it a ketone.
Example 4: CH₃COOH
This compound contains a carboxyl group (-COOH), classifying it as a carboxylic acid.
Example 5: CH₃COOCH₂CH₃
This compound features an ester group (-COO-), indicating it is an ester.
Example 6: CH₃CH₂NH₂
This compound has an amine group (-NH₂), therefore, it's an amine.
Example 7: CH₃CN
This compound possesses a cyano group (-CN), signifying it's a nitrile.
Example 8: C₆H₆
This is benzene, a classic example of an aromatic compound.
Example 9: CH₃CH₂Cl
This compound contains a chlorine atom (-Cl) bonded to a carbon, thus it's a halogenated compound.
Example 10: CH₃CH=CHCH₃
This compound contains a carbon-carbon double bond (C=C), classifying it as an alkene.
Example 11: CH₃C≡CH
This compound contains a carbon-carbon triple bond (C≡C), therefore, it's an alkyne.
Example 12: CH₃CH₂OCH₂CH₃
This compound has an ether group (-O-), identifying it as an ether.
Advanced Considerations and Complex Molecules
While these examples illustrate basic functional group identification, many organic molecules contain multiple functional groups. In such cases, identifying all present functional groups is crucial for understanding the compound's properties and reactivity. The order of precedence for naming may also play a role, especially in systematic nomenclature, where the principal functional group dictates the overall name of the compound.
For example, a molecule might contain both an alcohol and a carboxylic acid. In such a scenario, you would need to correctly identify both functional groups and understand their potential interactions.
Conclusion
Mastering the identification of functional groups is foundational to understanding organic chemistry. This guide provides a strong base for identifying common functional groups in various organic compounds. Through diligent practice and further study, you can develop proficiency in identifying even the most complex organic molecules and predicting their properties and reactions. Remember to always consider the context and potential interactions between multiple functional groups present in a molecule for a complete understanding.
Latest Posts
Latest Posts
-
What Is The Lightest Element On The Periodic Table
Mar 26, 2025
-
Which Of The Following Represent Statistical Information
Mar 26, 2025
-
Which Of The Following Is A Steroid Hormone
Mar 26, 2025
-
Osmosis Refers To The Movement Of
Mar 26, 2025
-
Digit 9 Is Always At Hundreds Place
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Identify The Functional Groups In The Following Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
