The Lower The Ph The Higher The Hydrogen Ion Concentration
News Leon
Mar 23, 2025 · 6 min read
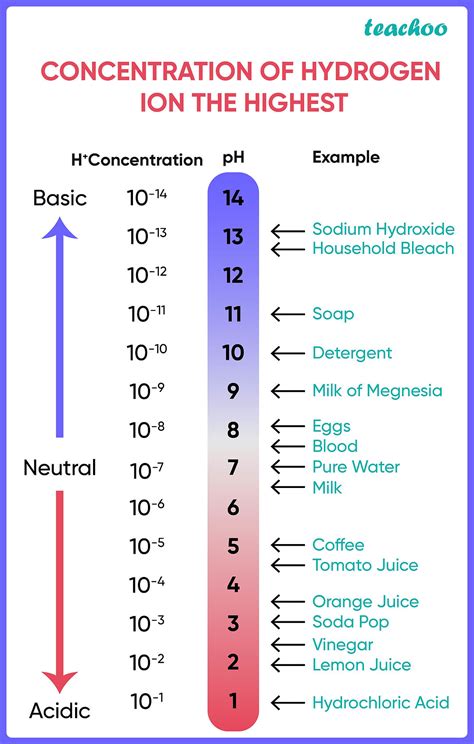
Table of Contents
The Lower the pH, the Higher the Hydrogen Ion Concentration: A Deep Dive into Acidity
The seemingly simple statement, "the lower the pH, the higher the hydrogen ion concentration," underpins a vast and crucial area of chemistry and biology. Understanding this fundamental relationship is key to comprehending numerous processes, from the corrosive nature of acids to the delicate balance of our internal bodily systems. This article will delve into the intricacies of pH, hydrogen ion concentration, and their implications across various scientific fields.
Understanding pH: A Measure of Acidity and Alkalinity
pH, a term derived from the French "pouvoir hydrogène" (power of hydrogen), quantifies the acidity or alkalinity of a solution. It's a logarithmic scale ranging from 0 to 14, where:
-
pH 7: Represents neutral solutions, with equal concentrations of hydrogen ions (H⁺) and hydroxide ions (OH⁻). Pure water at 25°C is a prime example.
-
pH < 7: Indicates acidic solutions, possessing a higher concentration of hydrogen ions (H⁺) than hydroxide ions (OH⁻). The lower the pH value, the stronger the acid.
-
pH > 7: Indicates alkaline (or basic) solutions, having a higher concentration of hydroxide ions (OH⁻) than hydrogen ions (H⁺). The higher the pH value, the stronger the base.
The logarithmic nature of the pH scale is crucial. A change of one pH unit represents a tenfold difference in hydrogen ion concentration. For instance, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4, and one hundred times more acidic than a solution with a pH of 5. This exponential relationship highlights the significant impact of even small pH changes.
The Role of Hydrogen Ions (H⁺)
Hydrogen ions, often represented as H⁺, are the key players determining a solution's pH. These ions are essentially protons, the positively charged components of hydrogen atoms that have lost their electrons. The concentration of these ions directly dictates the acidity of the solution. Strong acids readily release a high concentration of H⁺ ions into solution, while weak acids release fewer.
The Inverse Relationship: pH and Hydrogen Ion Concentration
The core principle—the lower the pH, the higher the hydrogen ion concentration—is directly linked to the mathematical definition of pH:
pH = -log₁₀[H⁺]
Where [H⁺] represents the molar concentration of hydrogen ions. The negative logarithm implies an inverse relationship. As [H⁺] increases, the logarithm becomes a larger positive number, but because of the negative sign, the pH value decreases. Conversely, as [H⁺] decreases, the pH value increases.
Mathematical Examples
Let's illustrate with examples:
-
Solution A: [H⁺] = 1 x 10⁻⁴ M (Molar) pH = -log₁₀(1 x 10⁻⁴) = 4
-
Solution B: [H⁺] = 1 x 10⁻² M pH = -log₁₀(1 x 10⁻²) = 2
Solution B, with a higher hydrogen ion concentration, has a lower pH value (2) compared to Solution A (pH 4). This clearly demonstrates the inverse relationship.
Implications Across Different Fields
The relationship between pH and hydrogen ion concentration has profound implications across numerous scientific and industrial fields:
1. Chemistry: Acid-Base Reactions and Equilibria
Understanding pH is fundamental to studying acid-base reactions and equilibria. The strength of an acid is directly related to its ability to donate H⁺ ions. pH measurements are crucial in titrations, determining the equivalence point, and understanding buffer solutions that resist changes in pH.
2. Biology: Maintaining Homeostasis
Biological systems are exquisitely sensitive to pH changes. The pH of blood, for example, must remain within a narrow range (around 7.4) for proper enzyme function and overall homeostasis. Deviations from this optimal range can have severe consequences, leading to acidosis or alkalosis. Biological buffers within the body help maintain a stable pH. The pH of different cellular compartments also plays a crucial role in various metabolic processes.
3. Environmental Science: Water Quality and Pollution
pH monitoring is essential in environmental science for assessing water quality. Acid rain, resulting from atmospheric pollutants, can significantly lower the pH of lakes and rivers, harming aquatic life. Soil pH also significantly impacts plant growth and nutrient availability. Monitoring pH helps assess the impact of pollution and manage environmental resources effectively.
4. Agriculture: Soil pH and Plant Growth
Optimal plant growth depends on the soil's pH. Different plants thrive in different pH ranges. Understanding and adjusting soil pH through fertilization and other techniques is critical for maximizing crop yields and ensuring healthy plant development.
5. Food Science: Food Preservation and Quality
pH plays a vital role in food preservation. Many food preservation techniques exploit the effects of acidity to inhibit microbial growth. Pickling, for example, relies on the low pH created by acids like vinegar to prevent spoilage. The pH of food also impacts its taste, texture, and overall quality.
6. Medicine: Drug Delivery and Physiological Processes
The pH of bodily fluids influences drug absorption, distribution, and metabolism. Many drugs are formulated with specific pH values to optimize their effectiveness and minimize side effects. Understanding pH is also crucial in diagnosing various medical conditions, like metabolic acidosis or alkalosis.
Measuring pH: Techniques and Applications
Several methods are used to measure pH, each suited to different applications:
1. pH Meters: Accurate and Versatile
pH meters are electronic instruments that provide precise pH readings. They use a pH-sensitive electrode (glass electrode) that generates a voltage proportional to the hydrogen ion concentration. pH meters are widely used in laboratories, industrial settings, and environmental monitoring.
2. pH Indicators: Simple and Visual
pH indicators are substances that change color over a specific pH range. They are typically used for less precise measurements or as a quick visual assessment of pH. Litmus paper, a common example, turns red in acidic solutions and blue in alkaline solutions. More sophisticated indicators, such as universal indicators, offer a wider range of color changes for a broader pH spectrum.
3. Spectrophotometry: Precise Quantitative Analysis
Spectrophotometry can be used to determine the pH of a solution by measuring the absorbance of a pH-sensitive dye at different wavelengths. This technique offers accurate and quantitative measurements, particularly for solutions with low concentrations of hydrogen ions.
Conclusion: The Significance of pH Understanding
The inverse relationship between pH and hydrogen ion concentration is a fundamental principle in chemistry and biology. Understanding this relationship is essential for comprehending numerous processes in various fields, from chemical reactions to biological functions and environmental monitoring. Accurate pH measurement techniques are crucial for various applications, ranging from scientific research to industrial processes and medical diagnostics. The ongoing research and development in pH measurement technologies continue to improve accuracy and expand its applications. This ensures a better understanding of our world and the systems that govern it, from the smallest biological processes to the largest ecological ones.
Latest Posts
Latest Posts
-
Which Of The Following Has The Shortest Bond Length
Mar 25, 2025
-
Do Prokaryotic Cells Have Membrane Bound Organelles
Mar 25, 2025
-
Which Of The Following Is An Input Device
Mar 25, 2025
-
Which Of The Following Is Not An Electromagnetic Wave
Mar 25, 2025
-
What Is The Molar Mass Of S
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about The Lower The Ph The Higher The Hydrogen Ion Concentration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
