The Graph Shows The Oxygen-binding Curves For Myoglobin And Hemoglobin
News Leon
Mar 21, 2025 · 6 min read
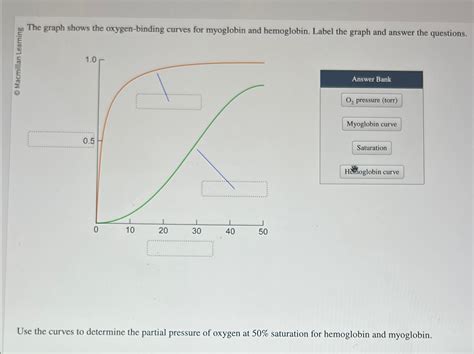
Table of Contents
The Graph Shows the Oxygen-Binding Curves for Myoglobin and Hemoglobin: A Deep Dive into Oxygen Transport
The image of oxygen-binding curves for myoglobin and hemoglobin is a cornerstone of understanding oxygen transport in the body. These curves, visually representing the relationship between partial pressure of oxygen (pO2) and oxygen saturation, reveal crucial differences in the functional roles of these two proteins. This article will delve deep into interpreting these curves, explaining the underlying mechanisms, and highlighting the physiological significance of their contrasting characteristics.
Understanding the Curves: A Visual Representation of Oxygen Binding
The graph typically depicts two sigmoid curves: one for hemoglobin (Hb) and one for myoglobin (Mb). The x-axis represents the partial pressure of oxygen (pO2), reflecting the amount of oxygen available in the surrounding environment, while the y-axis represents the fractional saturation of the protein with oxygen—the percentage of oxygen-binding sites occupied by oxygen molecules.
Myoglobin: The Oxygen Storage Champion
Myoglobin's curve is a simple hyperbolic curve. This means its oxygen binding follows a straightforward pattern: as pO2 increases, the oxygen saturation of myoglobin increases proportionally. Myoglobin has a high affinity for oxygen, meaning it readily binds oxygen even at low pO2 values. This high affinity makes myoglobin exceptionally efficient at storing oxygen within muscle tissue. At the pO2 levels found in resting muscle tissue, myoglobin remains largely saturated with oxygen, providing a readily available reservoir for oxygen when metabolic demand increases during exercise.
Hemoglobin: The Oxygen Transport Master
Hemoglobin's curve, on the other hand, is characterized by a sigmoidal shape. This S-shaped curve is not merely aesthetically different; it reflects the cooperative binding of oxygen to hemoglobin. This cooperative binding is a crucial feature that allows hemoglobin to effectively load oxygen in the lungs and unload it in the tissues.
- Cooperative Binding: Hemoglobin consists of four subunits, each with its own heme group capable of binding one oxygen molecule. The binding of the first oxygen molecule induces a conformational change in the hemoglobin molecule, increasing the affinity of the remaining subunits for oxygen. This positive cooperativity means that once one oxygen molecule binds, it becomes easier for subsequent molecules to bind. Conversely, the release of one oxygen molecule facilitates the release of others, resulting in efficient unloading in the tissues.
The Physiological Significance of the Differences
The differences between myoglobin and hemoglobin's oxygen-binding curves are directly related to their distinct physiological roles:
Myoglobin's Role in Oxygen Storage
The high affinity of myoglobin for oxygen ensures that it readily binds oxygen even at relatively low pO2 levels found in resting muscle tissue. This creates a reservoir of oxygen within muscle cells, ready to be released when oxygen demand increases during muscle contraction. When the pO2 in the muscle drops due to increased metabolic activity, myoglobin releases its stored oxygen to supply the working muscle. This ensures sustained muscle function even during periods of intense activity.
Hemoglobin's Role in Oxygen Transport
Hemoglobin's sigmoidal curve and cooperative binding allow it to perform its critical role of transporting oxygen from the lungs to the tissues. In the lungs, where pO2 is high (approximately 100 mmHg), hemoglobin becomes almost fully saturated with oxygen. As blood flows through the tissues, where pO2 is significantly lower (approximately 40 mmHg in resting tissue), the cooperative binding allows for efficient oxygen unloading. The steep portion of the curve in the physiological range ensures that a substantial amount of oxygen is released even with a relatively small drop in pO2.
Factors Affecting Hemoglobin's Oxygen Binding Curve
Several factors can shift the hemoglobin-oxygen dissociation curve, altering its affinity for oxygen and influencing oxygen delivery to the tissues. These include:
1. pH (The Bohr Effect):
A decrease in pH (increased acidity) shifts the curve to the right. This means that at any given pO2, hemoglobin's oxygen saturation is lower. This is known as the Bohr effect, and it's crucial for oxygen delivery to metabolically active tissues. During exercise, muscle cells produce lactic acid, lowering the pH. This rightward shift of the curve facilitates the release of more oxygen to the working muscles.
2. Carbon Dioxide (CO2):
Increased CO2 levels also shift the curve to the right, enhancing oxygen unloading in tissues with high metabolic activity, where CO2 production is elevated. CO2 binds to hemoglobin, forming carbaminohemoglobin, which reduces hemoglobin's affinity for oxygen.
3. 2,3-Bisphosphoglycerate (2,3-BPG):
2,3-BPG is a molecule found in red blood cells. It binds to hemoglobin, decreasing its affinity for oxygen and shifting the curve to the right. Levels of 2,3-BPG increase in conditions such as high altitude and during pregnancy, enhancing oxygen release to the tissues.
4. Temperature:
An increase in temperature shifts the curve to the right, reducing hemoglobin's affinity for oxygen. This is particularly relevant during exercise, where increased muscle activity leads to elevated temperatures and enhanced oxygen release to the working muscles.
Clinical Significance and Applications
Understanding the oxygen-binding curves of myoglobin and hemoglobin is crucial in various clinical settings:
- Diagnosis of Myocardial Infarction (Heart Attack): Damaged heart muscle releases myoglobin into the bloodstream. Measuring myoglobin levels can help in the early diagnosis of a heart attack.
- Blood Oxygenation Monitoring: Pulse oximetry indirectly measures the percentage of hemoglobin saturated with oxygen, providing a crucial indicator of blood oxygen levels.
- Understanding and Treating Hypoxia: Understanding the factors that shift the hemoglobin-oxygen dissociation curve allows clinicians to better manage conditions associated with hypoxia (low blood oxygen levels).
- Adaptation to High Altitude: The body's response to high altitude, including increased 2,3-BPG production, can be explained through the shifting of the oxygen-binding curve.
Beyond the Basics: Advanced Concepts
The simple representation of oxygen-binding curves can be further expanded upon. For example, the Hill coefficient, derived from the Hill equation, provides a quantitative measure of the cooperativity of oxygen binding to hemoglobin. A Hill coefficient greater than 1 indicates positive cooperativity. Furthermore, the intricacies of allosteric regulation, the role of specific amino acid residues in oxygen binding and release, and the dynamic interplay between hemoglobin and other blood components all contribute to the complexity and elegance of oxygen transport.
Conclusion
The oxygen-binding curves for myoglobin and hemoglobin provide a powerful visual representation of the crucial roles these proteins play in oxygen transport and storage. The hyperbolic curve of myoglobin highlights its function as an oxygen reservoir in muscle tissue, while the sigmoidal curve of hemoglobin, with its cooperative binding and sensitivity to various factors, underscores its role as the primary oxygen transporter in the blood. Understanding these curves and the factors that influence them is essential for comprehending fundamental physiological processes and their clinical implications. The differences between these two curves are not just graphical representations but reflect a finely tuned biological system ensuring efficient oxygen delivery throughout the body, adapting to varying metabolic demands and environmental conditions. Further research into the nuances of these proteins continues to reveal the intricacy and sophistication of the mechanisms involved in oxygen transport and utilization.
Latest Posts
Latest Posts
-
Ode To The West Wind Meaning
Mar 22, 2025
-
How Many Kilograms In 5000 Grams
Mar 22, 2025
-
Which Phase Of The Cardiac Cycle Is The Longest
Mar 22, 2025
-
The Energy Currency Used By Cells Is
Mar 22, 2025
-
What Is Group 17 On The Periodic Table Called
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about The Graph Shows The Oxygen-binding Curves For Myoglobin And Hemoglobin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
