Temperature And Kinetic Energy Are ___________ Proportional.
News Leon
Mar 15, 2025 · 5 min read
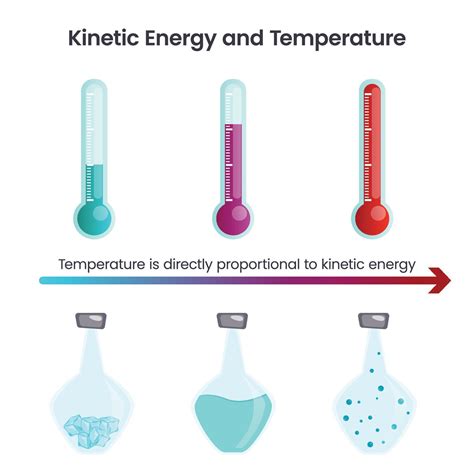
Table of Contents
Temperature and Kinetic Energy are Directly Proportional
The relationship between temperature and kinetic energy is fundamental to our understanding of thermodynamics and the behavior of matter. Simply put, temperature and kinetic energy are directly proportional. This means that as the temperature of a substance increases, the average kinetic energy of its particles also increases, and vice-versa. This seemingly simple statement underpins a vast array of physical phenomena, from the expansion of gases to the melting of solids. Let's delve deeper into this crucial relationship.
Understanding Kinetic Energy
Before exploring the connection between temperature and kinetic energy, it's crucial to define kinetic energy itself. Kinetic energy is the energy an object possesses due to its motion. For a single particle, this is expressed mathematically as:
KE = 1/2 * m * v²
where:
- KE represents kinetic energy
- m represents the mass of the particle
- v represents the velocity of the particle
This equation shows that kinetic energy is directly proportional to both the mass and the square of the velocity. A heavier object moving at the same speed as a lighter object will possess more kinetic energy. Similarly, an object moving at a higher speed will have significantly more kinetic energy than the same object moving slower.
Kinetic Energy at the Macroscopic Level
While the above equation applies to individual particles, understanding kinetic energy at the macroscopic level, i.e., for a collection of particles like those in a gas or liquid, requires a slightly different perspective. Instead of focusing on the kinetic energy of individual particles, we consider the average kinetic energy of all the particles within the system. This average kinetic energy is directly related to the temperature of the system.
The Link Between Temperature and Average Kinetic Energy
Temperature, unlike kinetic energy, isn't a direct measure of the energy of individual particles. Instead, temperature is a measure of the average kinetic energy of the particles in a substance. This is a crucial distinction. While individual particles may possess varying kinetic energies at any given moment due to collisions and interactions, the average kinetic energy provides a representative value reflecting the overall thermal state of the system.
The relationship between temperature and average kinetic energy can be expressed as:
KE<sub>avg</sub> ∝ T
where:
- KE<sub>avg</sub> represents the average kinetic energy of the particles
- T represents the absolute temperature (usually measured in Kelvin)
The proportionality symbol (∝) indicates a direct relationship. This means that if we double the absolute temperature, we double the average kinetic energy of the particles. If we halve the absolute temperature, we halve the average kinetic energy. This relationship holds true for ideal gases, and is a good approximation for many real-world substances, particularly at higher temperatures.
The Role of Absolute Temperature
It's important to note the use of absolute temperature (Kelvin) in this relationship. Unlike Celsius or Fahrenheit, the Kelvin scale starts at absolute zero, which is the theoretical point at which all particle motion ceases. Because of this, the Kelvin scale provides a direct proportionality to average kinetic energy; there's no offset or arbitrary zero point to account for.
Manifestations of the Direct Proportionality
The direct proportionality between temperature and kinetic energy has profound implications and manifests in various observable phenomena:
-
Thermal Expansion: As temperature increases, the average kinetic energy of particles increases. This leads to increased particle movement and greater inter-particle distances, resulting in thermal expansion of solids, liquids, and gases. This is why bridges and roads have expansion joints – to accommodate the changes in length due to temperature fluctuations.
-
Changes of State: The relationship between temperature and kinetic energy is central to phase transitions. Sufficient energy input (increasing temperature) can overcome the intermolecular forces holding particles together in a solid, leading to melting. Further increases in temperature and kinetic energy can then overcome the weaker intermolecular forces in a liquid, resulting in vaporization or boiling.
-
Gas Pressure: In gases, the pressure exerted on the container walls is a direct result of particle collisions. Higher temperature means higher average kinetic energy and more energetic collisions, leading to increased pressure. This is described by the Ideal Gas Law (PV = nRT), where temperature is directly related to pressure.
-
Rate of Chemical Reactions: Higher temperatures increase the kinetic energy of reactant molecules. This leads to more frequent and energetic collisions, increasing the likelihood of successful reactions and accelerating the overall rate of the chemical process. This is why many chemical reactions are faster at higher temperatures.
-
Diffusion and Brownian Motion: The random motion of particles, such as in diffusion or Brownian motion, is directly influenced by their kinetic energy. Higher temperatures lead to faster diffusion rates as particles move more rapidly.
Deviations from the Ideal Relationship
While the direct proportionality between temperature and kinetic energy is a useful approximation, it's essential to acknowledge some deviations from the ideal relationship:
-
Real Gases: Real gases deviate from ideal gas behavior, particularly at high pressures and low temperatures. Intermolecular forces and the finite size of gas molecules become significant, affecting the relationship between temperature and kinetic energy.
-
Quantum Effects: At extremely low temperatures, quantum mechanical effects become significant. The classical definition of kinetic energy may no longer accurately describe the behavior of particles, especially at the atomic and subatomic levels.
-
Specific Heat Capacity: Different substances have different specific heat capacities. This means that the same amount of heat energy input will result in different temperature changes for different substances. This is because some substances require more energy to raise the average kinetic energy of their particles than others.
Conclusion: A Cornerstone of Physics
The direct proportionality between temperature and kinetic energy is a cornerstone principle in physics and chemistry. It provides a fundamental framework for understanding a wide range of physical phenomena, from the expansion of materials to the rates of chemical reactions. While deviations from the ideal relationship exist, particularly under extreme conditions or with specific substances, the fundamental concept of directly proportional average kinetic energy to absolute temperature remains a powerful tool for explaining and predicting the behavior of matter. Understanding this connection is crucial for anyone pursuing studies in physics, chemistry, engineering, or related fields. Furthermore, this fundamental understanding empowers advancements in various fields, from designing more efficient engines to developing new materials with specific thermal properties. The ongoing research and refinement of our understanding of this relationship continues to be a driving force in scientific progress.
Latest Posts
Latest Posts
-
A Negatively Charged Ion Is Called
Mar 15, 2025
-
Two Different Isotopes Of An Element Have Different
Mar 15, 2025
-
If A Pea Plant Shows A Recessive Phenotype
Mar 15, 2025
-
Is A Patent A Current Asset
Mar 15, 2025
-
Why Are Producers So Important To An Ecosystem
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Temperature And Kinetic Energy Are ___________ Proportional. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
