Tap Water Pure Substance Or Mixture
News Leon
Apr 06, 2025 · 5 min read
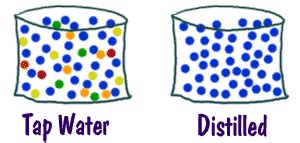
Table of Contents
Is Tap Water a Pure Substance or a Mixture? A Deep Dive into Water Composition
The question, "Is tap water a pure substance or a mixture?" seems simple at first glance. However, the answer delves into the fascinating world of chemistry and reveals the complexity of even something as commonplace as the water we drink daily. Understanding the composition of tap water is crucial for appreciating its properties and the processes involved in making it safe for consumption. This comprehensive guide will explore the scientific definition of pure substances and mixtures, analyze the components of tap water, and definitively answer the central question.
Pure Substances vs. Mixtures: A Fundamental Distinction
Before we delve into the specifics of tap water, let's establish a clear understanding of the core concepts: pure substances and mixtures.
Pure Substances: Defined
A pure substance is a form of matter that has a constant composition (a fixed ratio of elements) and consistent properties throughout the sample. It cannot be separated into simpler substances by physical methods. Pure substances can be further categorized into elements and compounds:
-
Elements: These are substances made up of only one type of atom. Examples include oxygen (O), hydrogen (H), and gold (Au). They cannot be broken down into simpler substances by chemical means.
-
Compounds: These are substances formed by the chemical combination of two or more elements in a fixed ratio. Water (H₂O), for example, is a compound consisting of two hydrogen atoms and one oxygen atom. Compounds can be broken down into their constituent elements through chemical processes.
Mixtures: Defined
A mixture, unlike a pure substance, consists of two or more substances physically combined. These substances retain their individual chemical properties and are not chemically bonded. Mixtures can be separated into their components using physical methods like filtration, distillation, or evaporation. Mixtures are further categorized into:
-
Homogeneous Mixtures: These mixtures have a uniform composition throughout. A saltwater solution is a prime example; the salt is evenly distributed throughout the water.
-
Heterogeneous Mixtures: These mixtures have a non-uniform composition, with different components visibly distinct. A mixture of sand and water is a classic example; the sand particles are clearly separated from the water.
The Composition of Tap Water: Unpacking the Ingredients
Now, let's turn our attention to the main subject: tap water. Is it a pure substance or a mixture? The answer is unequivocally: tap water is a mixture. While predominantly composed of water (H₂O), it contains numerous other substances, both dissolved and suspended. These can include:
1. Minerals: Essential and Otherwise
Tap water typically contains dissolved minerals, acquired during its journey through the ground and pipes. These minerals can include:
- Calcium (Ca): Contributes to water hardness.
- Magnesium (Mg): Another contributor to water hardness.
- Sodium (Na): Impacts taste and can be a concern for individuals on low-sodium diets.
- Potassium (K): Essential for various bodily functions.
- Iron (Fe): Can cause staining and discoloration if present in high concentrations.
- Manganese (Mn): Similar to iron, high levels can affect water quality.
- Fluoride (F): Often added intentionally to promote dental health.
The concentration of these minerals varies greatly depending on the water source and treatment processes.
2. Gases: Dissolved in Solution
Tap water also contains dissolved gases, primarily:
- Oxygen (O₂): Supports aquatic life.
- Carbon Dioxide (CO₂): Can contribute to acidity.
- Nitrogen (N₂): Generally inert in this context.
The levels of these gases depend on factors like temperature and atmospheric pressure.
3. Disinfection Byproducts: A Result of Treatment
To make tap water safe for consumption, water treatment plants often use disinfectants like chlorine or chloramine. These disinfectants react with organic matter in the water, creating disinfection byproducts (DBPs). These byproducts, while generally present in low concentrations, are subject to ongoing research and regulation due to potential health concerns.
4. Other Potential Contaminants: Organic and Inorganic
Depending on the source and treatment, tap water may also contain trace amounts of various other substances, including:
- Organic matter: From decaying vegetation or human waste.
- Pesticides: Runoff from agricultural lands.
- Pharmaceuticals: Traces of medications entering the water system.
- Heavy metals: From industrial pollution.
- Microbial contaminants: Bacteria, viruses, and parasites (ideally removed through treatment).
These contaminants are typically present in extremely low concentrations, but their presence underlines the complex nature of tap water.
Why Tap Water Isn't a Pure Substance: A Summary
The presence of dissolved minerals, gases, disinfection byproducts, and potentially other contaminants clearly demonstrates that tap water is a complex mixture. It's not a single chemical compound like pure water (H₂O), but rather a solution containing many different substances, each influencing its properties and characteristics. The relative amounts of these components are subject to significant variation based on location, season, and water treatment procedures.
The Importance of Water Treatment
Given that tap water is a mixture, understanding water treatment processes is essential for ensuring its safety and potability. Treatment facilities employ various techniques to remove or reduce the levels of harmful contaminants while maintaining essential minerals for taste and health benefits. These processes typically include:
- Coagulation and flocculation: Removing suspended solids.
- Sedimentation: Allowing solids to settle out.
- Filtration: Removing remaining particles.
- Disinfection: Killing harmful microorganisms.
The specific treatment methods depend on the quality of the raw water source and the regulatory requirements of the region.
The Role of Regulation in Ensuring Safe Tap Water
Government agencies play a vital role in monitoring and regulating the quality of tap water. They set standards for acceptable levels of contaminants and regularly test water samples to ensure compliance. These regulations are crucial for protecting public health and maintaining the safety of our drinking water supply.
Conclusion: Tap Water – A Vital Mixture
In conclusion, the question, "Is tap water a pure substance or a mixture?" is definitively answered: tap water is a mixture. While primarily composed of water (H₂O), the presence of various dissolved and suspended substances, both natural and man-made, fundamentally alters its composition. Understanding the multifaceted nature of tap water, its constituents, and the processes involved in making it safe for consumption allows us to appreciate the vital role of water treatment and regulation in protecting public health. The complexities of tap water highlight the intricate relationship between chemistry, environmental science, and public health. It's a testament to the importance of rigorous scientific understanding and responsible management of our shared resources.
Latest Posts
Latest Posts
-
What Is The Major Product Of This Reaction
Apr 08, 2025
-
Which Of The Following Is An Example Of Artificial Selection
Apr 08, 2025
-
How To Write A Matrix In Python
Apr 08, 2025
-
What Is The Largest Phylum Of Invertebrates
Apr 08, 2025
-
Do Cheek Cells Have A Nucleus
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Tap Water Pure Substance Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.