Sulphuric Acid Sodium Hydroxide Balanced Equation
News Leon
Apr 01, 2025 · 5 min read
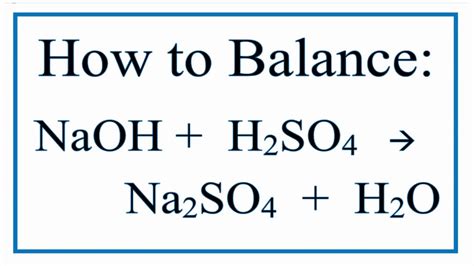
Table of Contents
The Balanced Equation of the Reaction Between Sulphuric Acid and Sodium Hydroxide: A Deep Dive
The reaction between sulfuric acid (H₂SO₄) and sodium hydroxide (NaOH) is a classic example of a neutralization reaction, a fundamental concept in chemistry. Understanding this reaction, including its balanced equation, stoichiometry, and applications, is crucial for students and professionals alike. This comprehensive guide will explore the reaction in detail, covering its balanced equation, the stoichiometry involved, the different types of reactions that can occur, and various real-world applications.
Understanding the Reactants
Before delving into the reaction itself, let's briefly examine the properties of the reactants: sulfuric acid and sodium hydroxide.
Sulfuric Acid (H₂SO₄): A strong mineral acid, sulfuric acid is highly corrosive and readily dissociates in water to produce hydrogen ions (H⁺) and sulfate ions (SO₄²⁻). Its high acidity makes it a powerful reactant in numerous chemical processes.
Sodium Hydroxide (NaOH): Also known as caustic soda or lye, sodium hydroxide is a strong base. It readily dissolves in water, dissociating into sodium ions (Na⁺) and hydroxide ions (OH⁻). Its strong basicity makes it equally reactive in various chemical applications.
The Balanced Chemical Equation
The reaction between sulfuric acid and sodium hydroxide is a neutralization reaction that produces water and a salt, sodium sulfate. The unbalanced equation is:
H₂SO₄ + NaOH → Na₂SO₄ + H₂O
This equation is unbalanced because the number of atoms of each element is not equal on both sides. To balance it, we need to ensure that the number of each type of atom is the same on the reactant and product sides. The balanced chemical equation is:
H₂SO₄ + 2NaOH → Na₂SO₄ + 2H₂O
This balanced equation shows that one molecule of sulfuric acid reacts with two molecules of sodium hydroxide to produce one molecule of sodium sulfate and two molecules of water. This 1:2 stoichiometric ratio is crucial for understanding the quantitative aspects of the reaction.
Stoichiometry and Calculations
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. The balanced equation allows us to perform various stoichiometric calculations. For instance, we can determine the amount of sodium sulfate produced from a given amount of sulfuric acid or sodium hydroxide, or vice versa.
Example Calculation:
Let's say we have 100 grams of sulfuric acid reacting with excess sodium hydroxide. How many grams of sodium sulfate will be produced?
-
Find the molar mass: The molar mass of H₂SO₄ is approximately 98 g/mol, and the molar mass of Na₂SO₄ is approximately 142 g/mol.
-
Convert grams to moles: 100 g H₂SO₄ / 98 g/mol = 1.02 moles H₂SO₄
-
Use the mole ratio: From the balanced equation, we know that 1 mole of H₂SO₄ produces 1 mole of Na₂SO₄. Therefore, 1.02 moles of H₂SO₄ will produce 1.02 moles of Na₂SO₄.
-
Convert moles back to grams: 1.02 moles Na₂SO₄ * 142 g/mol = 145 grams Na₂SO₄
Therefore, approximately 145 grams of sodium sulfate will be produced. This calculation demonstrates the power of the balanced equation in quantitative analysis.
Different Reaction Types and Considerations
While the primary reaction is a neutralization reaction, other aspects of the reaction deserve consideration. The reaction is exothermic, meaning it releases heat. The amount of heat released depends on the concentrations of the reactants and the conditions under which the reaction takes place. Careful handling is necessary due to the corrosive nature of both sulfuric acid and sodium hydroxide.
Furthermore, if the reactants are not carefully controlled, the reaction can be quite vigorous, potentially leading to splashing and other safety hazards. Therefore, appropriate safety precautions, such as the use of personal protective equipment (PPE) like gloves and eye protection, are essential when performing this reaction.
Applications of the Reaction
The reaction between sulfuric acid and sodium hydroxide has widespread applications across various industries:
-
Titration: This reaction is frequently used in titrations to determine the concentration of an unknown solution of either sulfuric acid or sodium hydroxide. By carefully measuring the volume of one reactant required to neutralize a known volume of the other, the unknown concentration can be precisely calculated.
-
Industrial Processes: In various industrial processes, this reaction is used for pH control and neutralization of waste streams. The precise control of pH is critical in many manufacturing processes.
-
Chemical Synthesis: Sodium sulfate, a product of this reaction, is used extensively in various industrial applications, including detergents, paper manufacturing, and textiles.
-
Laboratory Settings: The reaction serves as a valuable tool in chemistry labs for demonstrations, experiments, and quantitative analysis.
Beyond the Basics: Exploring Related Reactions
The fundamental reaction discussed above provides a strong foundation. However, understanding related concepts further enhances comprehension. Consider the reaction of sulfuric acid with other bases, or the reaction of sodium hydroxide with other acids. Exploring these related reactions helps build a comprehensive understanding of acid-base chemistry. For instance, the reaction of sulfuric acid with other hydroxides, like potassium hydroxide (KOH), follows a similar pattern, yielding water and the corresponding sulfate salt. Similarly, the reaction of sodium hydroxide with other acids will produce water and the respective salt.
Safety Precautions: A Crucial Note
Working with strong acids and bases like sulfuric acid and sodium hydroxide requires meticulous attention to safety. Always wear appropriate personal protective equipment (PPE), including safety glasses, gloves, and a lab coat. Work in a well-ventilated area, and ensure proper disposal of waste chemicals according to safety guidelines. Never directly handle concentrated acids or bases without proper training and safety precautions. Consult relevant safety data sheets (SDS) for detailed safety information before handling these chemicals.
Conclusion: A Foundation for Further Learning
The neutralization reaction between sulfuric acid and sodium hydroxide is a cornerstone of chemistry. Understanding its balanced equation, stoichiometry, applications, and safety considerations is vital for anyone working with these chemicals. This comprehensive exploration serves as a robust foundation for further learning and deeper exploration of acid-base chemistry and its numerous applications in various scientific and industrial fields. Remember to always prioritize safety when working with chemicals, and consult relevant safety data sheets (SDS) before starting any experiment. The balanced equation, H₂SO₄ + 2NaOH → Na₂SO₄ + 2H₂O, is not just a formula; it's a gateway to understanding the intricate world of chemical reactions and their significant impact on our world.
Latest Posts
Latest Posts
-
The Product Of Two Irrational Numbers Is Always Irrational
Apr 02, 2025
-
Which Statement About Exothermic Reactions Is Accurate
Apr 02, 2025
-
A Lens Produces A Real Image Of A Real Object
Apr 02, 2025
-
Explain Common Different And Conflicting Goals By Giving Appropriate Examples
Apr 02, 2025
-
What Is The Major Product For The Following Reaction Sequence
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Sulphuric Acid Sodium Hydroxide Balanced Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
