Si Unit For Density Of Water
News Leon
Mar 23, 2025 · 5 min read
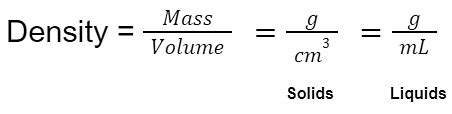
Table of Contents
The SI Unit for Density of Water: A Deep Dive
The density of water, a seemingly simple concept, plays a crucial role in numerous scientific fields, from chemistry and physics to engineering and environmental science. Understanding its measurement, specifically within the International System of Units (SI), is fundamental to accurate calculations and reliable experimental results. This article will delve into the SI unit for density, its application to water, and the nuances associated with precise measurements. We'll explore the factors affecting water density and the importance of standardized units in scientific rigor.
Understanding Density and its SI Unit
Density is a fundamental physical property defined as the mass per unit volume of a substance. It essentially describes how tightly packed the matter is within a given space. The SI unit for density is kilograms per cubic meter (kg/m³). This unit is derived from the base SI units of mass (kilogram) and length (meter). While other units like grams per cubic centimeter (g/cm³) are commonly used, they are not the official SI unit and require conversion for consistency in scientific calculations.
Why kg/m³ is the Preferred Unit
Using kg/m³ as the standard offers several advantages:
- Consistency: It maintains consistency with the broader SI system, simplifying calculations and preventing conversion errors.
- Clarity: It unambiguously defines the mass and volume units used.
- Scalability: It works well across a wide range of densities, from extremely dense materials to gases.
While g/cm³ might seem more intuitive for everyday applications, sticking to kg/m³ ensures global standardization and avoids potential confusion in complex scientific work.
Density of Water: Variations and Factors
The density of water is not a constant; it varies depending on several factors:
-
Temperature: This is the most significant factor. Water's density is highest at 4 °C (39.2 °F), reaching approximately 999.97 kg/m³. Above and below this temperature, the density decreases. This anomalous behavior of water is crucial for aquatic life, as it prevents bodies of water from freezing solid from the bottom up.
-
Pressure: Increasing pressure increases the density of water, though the effect is relatively minor compared to the effect of temperature at typical environmental pressures. This becomes more significant at extreme depths in the ocean.
-
Salinity: Saltwater has a higher density than freshwater due to the dissolved salts. The density increases proportionally with the concentration of dissolved salts. Oceanographers meticulously measure salinity to accurately determine seawater density.
-
Isotopic Composition: The relative abundance of different isotopes of hydrogen and oxygen in water molecules slightly affects the density. This effect is usually small but can be relevant in highly precise measurements.
Precise Measurement of Water Density
Accurately measuring the density of water requires precise instrumentation and controlled conditions. Common methods include:
-
Pycnometry: This classic method involves carefully weighing a known volume of water using a pycnometer, a specialized volumetric flask. The density is calculated by dividing the mass of water by its volume. The precision of pycnometry is high, provided the temperature is carefully controlled.
-
Hydrometry: Hydrometers, also known as density meters, directly measure the density of a liquid based on its buoyancy. These are widely used in various industries for quick density checks, but their accuracy might be lower than pycnometry for precise scientific work.
-
Digital Density Meters: These sophisticated instruments use advanced techniques like oscillating U-tubes or vibrating elements to determine the density with high accuracy and precision. They are often equipped with temperature compensation, ensuring reliable results.
Importance of Temperature Control
Maintaining a precise temperature is critical for accurate density measurements. Even small temperature fluctuations can lead to significant errors in the calculated density. Therefore, temperature-controlled environments or water baths are essential when conducting these measurements.
Applications of Water Density Data
Understanding the precise density of water has far-reaching applications across various disciplines:
-
Oceanography: Density differences drive ocean currents, which play a significant role in global climate patterns and marine ecosystems. Oceanographers use density data to model ocean circulation and understand its impact on marine life and the global climate.
-
Hydrology: In hydrology, density plays a role in understanding water flow in rivers, groundwater movement, and the behavior of water in soils. This is crucial for managing water resources, predicting floods, and designing irrigation systems.
-
Meteorology: The density of water vapor in the atmosphere affects weather patterns, cloud formation, and precipitation. Accurate density data contributes to improved weather forecasting models.
-
Chemistry and Physics: Density measurements are essential in various chemical and physical experiments and calculations, including determining the concentration of solutions and studying the behavior of fluids.
-
Engineering: Engineers utilize density data in designing hydraulic systems, pipelines, and other applications involving water flow and pressure.
Conclusion: The Significance of Standardized Units
The SI unit for the density of water, kg/m³, provides a crucial foundation for accurate scientific measurement and consistent results across various fields. Understanding the factors affecting water density—primarily temperature, pressure, and salinity—is essential for obtaining reliable data. The use of standardized units, along with precise measurement techniques and strict temperature control, ensures that research findings are replicable and globally comparable. This contributes significantly to advancing scientific knowledge and facilitating technological innovation in areas heavily reliant on accurate water density data.
Further Exploration
For those interested in delving deeper, researching specific applications of water density within various scientific disciplines is highly recommended. Exploring the underlying principles of density measurement techniques and the development of increasingly precise instruments would provide a more comprehensive understanding of this fundamental property of water. The impact of water density on environmental processes and ecological systems also warrants further investigation. Additionally, researching the history of density measurement and the evolution of standardized units would further enhance comprehension of this crucial aspect of scientific methodology.
Latest Posts
Latest Posts
-
The Current Density Inside A Long Solid Cylindrical Wire
Mar 24, 2025
-
Which Statement Best Describes The Passage
Mar 24, 2025
-
How Many Chromosomes Does A Zebra Have
Mar 24, 2025
-
How Many Electrons Does Carbon 12 Have
Mar 24, 2025
-
In The Figure Two Tiny Conducting Balls
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Si Unit For Density Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
