How Many Electrons Does Carbon 12 Have
News Leon
Mar 24, 2025 · 5 min read
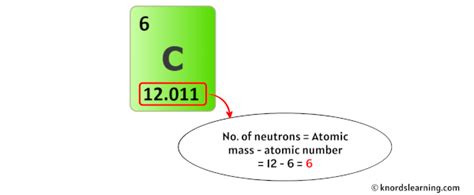
Table of Contents
How Many Electrons Does Carbon-12 Have? A Deep Dive into Atomic Structure
Understanding the number of electrons in an atom is fundamental to comprehending chemistry and physics. This article delves into the specifics of Carbon-12, explaining not only its electron count but also exploring the underlying principles of atomic structure, isotopic variations, and the significance of electron configuration.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we answer the central question, let's establish a foundational understanding of atomic structure. An atom is the basic unit of matter, consisting of three primary subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element; all carbon atoms have 6 protons.
- Neutrons: Neutrally charged particles also residing in the nucleus. The number of neutrons can vary within an element, leading to isotopes (discussed later).
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom, ensuring an overall neutral charge.
Carbon-12: Isotopes and Atomic Mass
The "12" in Carbon-12 refers to its mass number, which represents the total number of protons and neutrons in the nucleus. Since all carbon atoms have 6 protons, Carbon-12 has 6 neutrons (12 - 6 = 6). This is the most abundant and stable isotope of carbon.
It's crucial to understand the concept of isotopes. Isotopes are atoms of the same element (same number of protons) but with differing numbers of neutrons. While Carbon-12 is the most common, other isotopes exist, such as Carbon-13 (6 protons, 7 neutrons) and Carbon-14 (6 protons, 8 neutrons). These isotopes have the same number of electrons as Carbon-12 when neutral.
Determining the Number of Electrons in Carbon-12
In a neutral atom, the number of electrons is equal to the number of protons. Therefore, a neutral Carbon-12 atom has 6 electrons. This equality of positive and negative charges results in an overall electrically neutral atom. This fundamental principle applies to all neutral atoms of any element.
However, atoms can gain or lose electrons, becoming charged ions. If a carbon atom loses electrons, it becomes a positively charged ion (cation), and if it gains electrons, it becomes a negatively charged ion (anion). The number of electrons would then differ from the number of protons.
Electron Configuration and Shells
Electrons don't just randomly orbit the nucleus. They occupy specific energy levels or shells around the nucleus. These shells have a maximum capacity for electrons. The electron configuration describes how electrons are distributed among these shells. For carbon, the electron configuration is 1s²2s²2p².
- 1s²: The first shell (n=1) can hold a maximum of two electrons, and both are in the s subshell.
- 2s²: The second shell (n=2) also has an s subshell which holds two electrons.
- 2p²: The second shell also contains a p subshell, which can hold up to six electrons, but in carbon, only two electrons occupy this subshell.
This electron configuration is crucial for understanding the chemical behavior of carbon, its ability to form four covalent bonds, and its role in organic chemistry. The valence electrons, those in the outermost shell (2s²2p²), are particularly important in chemical bonding.
The Significance of Carbon-12 in Science
Carbon-12 holds a special place in science due to its role as the standard for atomic mass. The atomic mass unit (amu) is defined as one-twelfth the mass of a single Carbon-12 atom. This definition provides a consistent and accurate basis for measuring the mass of other atoms and molecules.
The abundance and stability of Carbon-12 make it an ideal choice for this standard. Its use simplifies calculations in chemistry and physics, ensuring consistent results across various experiments and analyses.
Carbon-12 and Nuclear Reactions
While the number of electrons largely determines the chemical properties of an element, the nucleus (protons and neutrons) plays a crucial role in nuclear reactions. Although Carbon-12 is relatively stable, it can participate in nuclear reactions, including fusion and fission, under specific high-energy conditions. These reactions alter the nuclear composition, possibly changing the number of protons and neutrons but not necessarily the number of electrons (initially).
Carbon-12 in Organic Chemistry and Biology
Carbon's unique ability to form four covalent bonds is the foundation of organic chemistry and the immense diversity of organic molecules found in living organisms. The tetrahedral structure resulting from these bonds allows carbon to form long chains, branched structures, and rings, leading to the vast array of molecules necessary for life. This bonding capability, intimately linked to its electron configuration, is a key factor in the biological significance of carbon.
Carbon Dating and Carbon-14
While Carbon-12 is stable, Carbon-14, a radioactive isotope of carbon, is used in radiocarbon dating. The decay rate of Carbon-14 allows scientists to estimate the age of organic materials, providing valuable insights into archaeology, geology, and other fields. This illustrates the importance of understanding isotopic variations of carbon.
Beyond the Basics: Ions and Charged Carbon
As previously mentioned, a carbon atom can lose or gain electrons, forming ions. For example, a carbon atom could theoretically lose four electrons, resulting in a +4 ion (C⁴⁺), or gain four electrons, forming a -4 ion (C⁴⁻). However, these highly charged ions are not commonly observed under normal conditions.
More commonly, carbon forms covalent bonds, sharing electrons with other atoms rather than completely transferring electrons to create ions. This sharing of electrons results in stable molecules.
Conclusion: The Importance of Understanding Atomic Structure
Determining the number of electrons in Carbon-12 – six, in a neutral atom – is a seemingly simple question that opens up a vast understanding of atomic structure, chemical bonding, isotopic variations, and the significance of this crucial element in both the natural world and scientific research. From its role as the standard for atomic mass to its crucial function in organic chemistry and biology, Carbon-12 continues to be a fundamental element for exploration and understanding. Mastering the basic principles discussed here lays the foundation for deeper explorations into chemistry, physics, and related fields.
Latest Posts
Latest Posts
-
A Convex Lens Of Focal Length 10cm
Mar 26, 2025
-
Gases Have Indefinite Shape And Volume
Mar 26, 2025
-
Round 64 To The Nearest Ten
Mar 26, 2025
-
Which Of The Following Are Meso Compounds
Mar 26, 2025
-
Words Beginning With The Same Letter
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Carbon 12 Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
