Oxidation State Of O In H2o
News Leon
Mar 16, 2025 · 6 min read
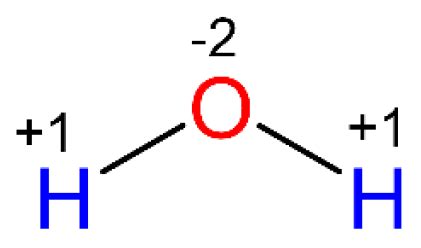
Table of Contents
The Oxidation State of Oxygen in Water (H₂O): A Deep Dive
The seemingly simple molecule of water, H₂O, provides a fascinating case study in understanding oxidation states. While the concept might seem straightforward at first glance, a deeper exploration reveals nuances and subtleties that are crucial for comprehending chemical reactions and bonding. This article will delve into the oxidation state of oxygen in water, exploring the underlying principles, common misconceptions, and its implications in various chemical contexts.
Understanding Oxidation States
Before directly addressing the oxidation state of oxygen in water, let's establish a firm foundation on the concept itself. Oxidation state, also known as oxidation number, is a number assigned to an atom in a molecule or ion that represents the hypothetical charge the atom would have if all bonds to atoms of different elements were 100% ionic. It's a crucial tool for understanding electron transfer in chemical reactions, particularly in redox (reduction-oxidation) processes.
Several rules govern the assignment of oxidation states:
-
Free elements: The oxidation state of an atom in its elemental form is always 0. For example, the oxidation state of oxygen in O₂ is 0, and the oxidation state of hydrogen in H₂ is 0.
-
Monatomic ions: The oxidation state of a monatomic ion is equal to its charge. For instance, the oxidation state of Na⁺ is +1, and the oxidation state of Cl⁻ is -1.
-
Fluorine: Fluorine, the most electronegative element, always has an oxidation state of -1 in its compounds.
-
Hydrogen: Hydrogen typically has an oxidation state of +1 in its compounds, except when bonded to metals (like in metal hydrides), where it has an oxidation state of -1.
-
Oxygen: Oxygen typically has an oxidation state of -2 in its compounds, except in peroxides (like H₂O₂), where it has an oxidation state of -1, and in compounds with fluorine (like OF₂), where it has a positive oxidation state.
-
The sum of oxidation states: In a neutral molecule, the sum of the oxidation states of all atoms must be 0. In a polyatomic ion, the sum of the oxidation states must equal the charge of the ion.
Determining the Oxidation State of Oxygen in H₂O
Applying these rules to water (H₂O), we can determine the oxidation state of oxygen. Hydrogen, being less electronegative than oxygen, typically exhibits an oxidation state of +1. Since water is a neutral molecule, the sum of the oxidation states must be 0.
Let's represent the oxidation state of oxygen as 'x'. We have two hydrogen atoms (+1 each) and one oxygen atom (x). Therefore, the equation is:
2(+1) + x = 0
Solving for x:
x = -2
Therefore, the oxidation state of oxygen in water is -2. This signifies that, in the simplified ionic model, oxygen in water has gained two electrons. It's crucial to remember that this is a formal assignment; the bond between hydrogen and oxygen in water is significantly covalent, not purely ionic.
Misconceptions about Oxidation States in Water
A common misconception is that the oxidation state directly reflects the actual charge on an atom. This is incorrect, especially in covalent compounds like water. The oxidation state is a formal assignment based on a hypothetical ionic model, which simplifies the electron distribution for bookkeeping purposes. The actual electron distribution in water is much more complex due to the covalent nature of the O-H bonds.
Another misconception is that the oxidation state remains constant regardless of the chemical environment. While the oxidation state of oxygen in most of its compounds is -2, there are exceptions, as previously mentioned, with peroxides and compounds with fluorine. Understanding these exceptions is essential for accurately predicting and interpreting redox reactions.
The Significance of Oxygen's Oxidation State in Water
The -2 oxidation state of oxygen in water is fundamental to understanding its role in various chemical processes. This oxidation state influences its reactivity, making it a crucial participant in redox reactions. For instance:
-
Photosynthesis: In photosynthesis, water molecules are oxidized, meaning oxygen loses electrons, increasing its oxidation state. This process is vital for the production of oxygen in the atmosphere and the conversion of light energy into chemical energy in plants.
-
Respiration: In cellular respiration, oxygen acts as an oxidizing agent, accepting electrons from other molecules and reducing its oxidation state. This process releases energy vital for life.
-
Corrosion: Water plays a significant role in corrosion processes, where metals are oxidized, and oxygen is reduced. Understanding the oxidation states involved is crucial for designing corrosion-resistant materials and developing effective corrosion prevention strategies.
-
Combustion: The combustion of fuels involves the oxidation of the fuel by oxygen, resulting in the release of energy. The oxidation state of oxygen changes during combustion, leading to the formation of various oxidation products such as carbon dioxide and water.
-
Electrochemistry: Electrochemical processes, such as batteries and fuel cells, rely on redox reactions. Understanding the oxidation states of the involved species, including oxygen in water, is essential for designing and optimizing electrochemical devices.
Advanced Considerations: Beyond the Simple Model
While the -2 oxidation state provides a useful simplification, a more sophisticated understanding requires considering the actual electron distribution in the water molecule. Molecular orbital theory reveals that the oxygen atom in water possesses a partial negative charge due to its higher electronegativity, while the hydrogen atoms possess partial positive charges. This unequal charge distribution is responsible for the polar nature of water, a property that underpins its unique solvent properties and biological importance.
Furthermore, advanced techniques like X-ray photoelectron spectroscopy (XPS) can provide experimental data on the electron binding energies of atoms in molecules, offering a more accurate picture of the electron distribution than the simplistic oxidation state model.
Conclusion: A Versatile Tool in Chemistry
The oxidation state of oxygen in water, while a seemingly simple concept, serves as a powerful tool for understanding chemical bonding and reactivity. While the -2 oxidation state is a helpful simplification, it's crucial to remember its limitations and appreciate the more nuanced picture provided by advanced chemical theories and experimental techniques. Understanding the oxidation state of oxygen in water is fundamental to comprehending a wide range of chemical processes and their impact on our world, from the biological processes sustaining life to the industrial processes shaping our society. By grasping this concept fully, we unlock a deeper understanding of the fundamental principles governing the behavior of matter. The exploration of oxidation states provides a pathway to more intricate chemical concepts and enhances our ability to predict and interpret chemical reactions with increased accuracy. It is a cornerstone of chemical knowledge, paving the way for more sophisticated explorations in the field.
Latest Posts
Latest Posts
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
-
Which Is The Correct Order Of The Scientific Method
Mar 17, 2025
-
How Long Is A Thousand Days
Mar 17, 2025
-
How Many Valence Electrons In Copper
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Oxidation State Of O In H2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
