Oxidation Number Of Manganese In Kmno4
News Leon
Mar 16, 2025 · 5 min read
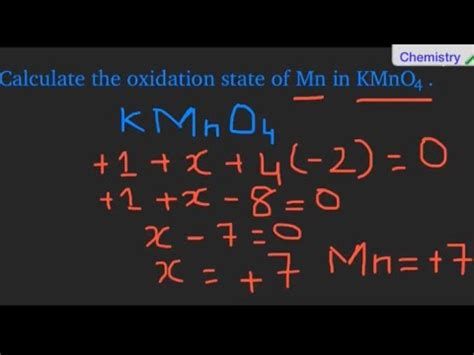
Table of Contents
Understanding the Oxidation Number of Manganese in KMnO₄
Potassium permanganate (KMnO₄) is a vibrant purple, crystalline compound frequently used as a strong oxidizing agent in various chemical reactions and applications. Understanding the oxidation state of manganese (Mn) within this compound is crucial for comprehending its reactivity and predicting its behavior in redox reactions. This article delves deep into determining and explaining the oxidation number of manganese in KMnO₄, exploring related concepts and applications.
Determining the Oxidation Number of Manganese
The oxidation number, also known as the oxidation state, represents the hypothetical charge an atom would have if all bonds to atoms of different elements were completely ionic. Assigning oxidation numbers follows a set of rules, allowing us to systematically determine the oxidation state of each element in a compound. Let's apply these rules to KMnO₄:
Rules for Assigning Oxidation Numbers
-
The oxidation number of an uncombined element is always zero. For example, the oxidation number of elemental manganese (Mn) is 0.
-
The oxidation number of a monatomic ion is equal to its charge. For example, the oxidation number of Cl⁻ is -1, and the oxidation number of Na⁺ is +1.
-
The oxidation number of hydrogen is +1, except in metal hydrides where it is -1. Examples include H₂O (hydrogen is +1) and NaH (hydrogen is -1).
-
The oxidation number of oxygen is usually -2, except in peroxides (like H₂O₂) where it is -1, and in superoxides where it is -1/2.
-
The sum of the oxidation numbers of all atoms in a neutral compound is zero.
-
The sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion.
Applying the Rules to KMnO₄
Let's assign oxidation numbers to each element in KMnO₄:
-
Potassium (K): Potassium is an alkali metal, and alkali metals always have an oxidation number of +1.
-
Oxygen (O): Oxygen typically has an oxidation number of -2 (excluding exceptions mentioned above). Since there are four oxygen atoms in KMnO₄, the total contribution from oxygen is 4 * (-2) = -8.
-
Manganese (Mn): Let's denote the oxidation number of manganese as 'x'.
Now, applying rule 5 (the sum of oxidation numbers in a neutral compound is zero):
(+1) + x + 4(-2) = 0
Solving for x:
1 + x - 8 = 0
x = +7
Therefore, the oxidation number of manganese (Mn) in KMnO₄ is +7.
Significance of the +7 Oxidation State of Manganese
The +7 oxidation state is the highest oxidation state manganese can achieve. This high oxidation state indicates that manganese in KMnO₄ is highly electronegative, readily accepting electrons. This property is the reason behind KMnO₄'s potent oxidizing power.
Implications for Redox Reactions
Because manganese in KMnO₄ has a +7 oxidation state, it readily accepts electrons during redox reactions, undergoing reduction. This reduction can lead to several different manganese oxidation states depending on the reaction conditions (pH, presence of other reducing agents, etc.). Common reduction products include MnO₂ (Mn has an oxidation state of +4), Mn²⁺ (Mn has an oxidation state of +2), and even elemental manganese (Mn with an oxidation state of 0).
The variety of reduction products makes KMnO₄ a versatile reagent in titrations and other redox reactions. The specific reduction product formed depends heavily on the reaction environment and the reducing agent used.
Applications of KMnO₄ based on its Oxidation State
The unique +7 oxidation state of manganese in KMnO₄ makes it highly useful in a wide range of applications:
1. Oxidizing Agent in Titrations
KMnO₄ is a popular titrant in redox titrations. Its intense purple color disappears upon reduction, providing a self-indicating endpoint. This makes it particularly convenient for determining the concentration of various reducing agents. Examples include:
-
Determining the concentration of iron(II) ions: In acidic conditions, KMnO₄ oxidizes Fe²⁺ to Fe³⁺, itself being reduced to Mn²⁺.
-
Determining the concentration of oxalic acid: KMnO₄ oxidizes oxalic acid (C₂H₂O₄) to carbon dioxide (CO₂) in acidic conditions.
-
Determining the concentration of hydrogen peroxide: KMnO₄ oxidizes hydrogen peroxide (H₂O₂) to oxygen (O₂).
The stoichiometry of these reactions is directly related to the change in the oxidation state of manganese, making the calculations straightforward.
2. Disinfectant and Water Purifier
KMnO₄'s strong oxidizing power makes it effective as a disinfectant and water purifier. It kills bacteria and other microorganisms by oxidizing their essential components. It is often used to disinfect drinking water in emergency situations or in areas with limited access to clean water sources. However, its use requires careful control as excess KMnO₄ can be harmful.
3. Synthesis of Organic Compounds
KMnO₄ is used in organic chemistry as a strong oxidizing agent in various syntheses. It can oxidize alcohols to aldehydes or ketones, alkenes to diols, and other functional groups depending on reaction conditions. The selectivity of oxidation can sometimes be controlled by adjusting the pH and reaction temperature.
4. Analytical Chemistry
Beyond its use in titrations, KMnO₄ has other analytical applications. It can be used in qualitative analysis to detect certain substances based on their ability to be oxidized by KMnO₄. The color change serves as a visual indicator.
5. Medicinal Applications
Historically, KMnO₄ had some medicinal applications, particularly as an antiseptic and disinfectant for minor wounds. However, its use in this context is declining due to safety concerns and the availability of more effective and less toxic alternatives.
Safety Precautions when Handling KMnO₄
KMnO₄ is a powerful oxidizer and should be handled with care. It can cause skin and eye irritation, and ingestion can be dangerous. Always wear appropriate personal protective equipment (PPE), including gloves, eye protection, and a lab coat when handling KMnO₄. Proper storage in a cool, dry place away from flammable materials is crucial to prevent accidents.
Conclusion: The Versatile Role of Manganese's +7 Oxidation State
The +7 oxidation state of manganese in KMnO₄ is the key to its diverse applications. This high oxidation state confers strong oxidizing power, making it a valuable reagent in various chemical processes, from titrations to water purification and organic synthesis. Understanding the oxidation number and its implications is essential for safely and effectively utilizing this versatile compound in various fields of chemistry. Further research and development might uncover even more applications leveraging this unique chemical property. The strong oxidizing potential, coupled with the visual indicator aspect in titrations, makes KMnO₄ a crucial chemical in both educational and industrial settings. However, always remember to prioritize safety and handle this powerful chemical with the necessary precautions.
Latest Posts
Latest Posts
-
Is Boiling Water A Physical Change
Mar 17, 2025
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
-
Which Is The Correct Order Of The Scientific Method
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Oxidation Number Of Manganese In Kmno4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
