Oxidation Number Of I In Io3
News Leon
Mar 30, 2025 · 5 min read
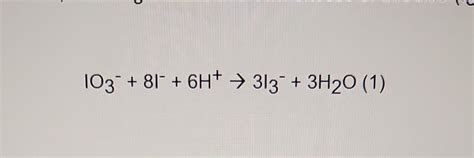
Table of Contents
Determining the Oxidation Number of Iodine in IO₃⁻
The determination of oxidation numbers is a fundamental concept in chemistry, crucial for balancing redox reactions and understanding the reactivity of chemical species. This article delves into the method for calculating the oxidation number of iodine (I) in the iodate ion (IO₃⁻), providing a comprehensive explanation and exploring related concepts. We'll also touch upon the significance of oxidation numbers in various chemical contexts.
Understanding Oxidation Numbers
Before we dive into the calculation for IO₃⁻, let's establish a solid understanding of what oxidation numbers represent. The oxidation number, also known as the oxidation state, is a number assigned to an atom in a molecule or ion that represents its apparent charge. It's a bookkeeping tool that helps us track electron transfer in chemical reactions. It's important to note that the oxidation number doesn't necessarily represent the actual charge on an atom; it's a formal charge assigned based on a set of rules.
Rules for Assigning Oxidation Numbers
Several rules guide the assignment of oxidation numbers. These rules are hierarchical; if a rule conflicts with another, the higher-priority rule takes precedence. The key rules include:
-
Rule 1: The oxidation number of an atom in its elemental form is always zero. For example, the oxidation number of O₂ is 0, and the oxidation number of I₂ is 0.
-
Rule 2: The oxidation number of a monatomic ion is equal to its charge. For example, the oxidation number of Na⁺ is +1, and the oxidation number of Cl⁻ is -1.
-
Rule 3: The oxidation number of hydrogen is +1, except in metal hydrides where it is -1. In most compounds, hydrogen acts as a +1 cation. However, in metal hydrides like NaH, hydrogen exists as a -1 anion.
-
Rule 4: The oxidation number of oxygen is usually -2, except in peroxides where it is -1 and in superoxides where it is -1/2. This is a crucial rule for determining oxidation numbers in many compounds, including the iodate ion.
-
Rule 5: The sum of the oxidation numbers of all atoms in a neutral molecule is zero. This rule ensures the overall neutrality of the molecule.
-
Rule 6: The sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion. This is the key rule we will apply to calculate the oxidation number of iodine in IO₃⁻.
Calculating the Oxidation Number of Iodine in IO₃⁻
Now, let's apply these rules to determine the oxidation number of iodine in the iodate ion (IO₃⁻).
-
Identify the known oxidation numbers: We know that the oxidation number of oxygen is typically -2 (Rule 4).
-
Consider the overall charge: The iodate ion carries a -1 charge.
-
Set up an algebraic equation: Let 'x' represent the oxidation number of iodine. We can write the equation based on Rule 6:
x + 3(-2) = -1
-
Solve for x:
x - 6 = -1 x = +5
Therefore, the oxidation number of iodine in IO₃⁻ is +5.
Significance of the +5 Oxidation State of Iodine
The +5 oxidation state of iodine in IO₃⁻ is quite significant. Iodine exhibits a wide range of oxidation states, from -1 to +7, showcasing its versatility in chemical reactions. The +5 state represents a relatively high oxidation state for iodine, implying that iodine has undergone significant oxidation. This high oxidation state contributes to the strong oxidizing properties of iodate.
Iodate as an Oxidizing Agent
The high oxidation state of iodine in IO₃⁻ makes the iodate ion a powerful oxidizing agent. It readily accepts electrons, causing the reduction of iodine to a lower oxidation state. This property is exploited in various applications, including:
-
Analytical Chemistry: Iodate is used in redox titrations to determine the concentration of reducing agents.
-
Organic Chemistry: Iodate can be used as an oxidizing agent in organic synthesis.
-
Industrial Applications: Iodate finds use in various industrial processes requiring strong oxidizing agents.
Other Iodine Oxidation States
It's important to note that iodine can exist in other oxidation states. For instance, in iodide (I⁻), iodine has an oxidation state of -1. In iodine monochloride (ICl), iodine exhibits a +1 oxidation state. In periodate (IO₄⁻), iodine displays a +7 oxidation state. Each oxidation state corresponds to a different chemical reactivity and potential applications.
Advanced Concepts: Beyond Simple Calculations
While the basic calculation method for IO₃⁻ is straightforward, understanding the nuances of oxidation number assignment becomes more crucial when dealing with complex molecules and compounds.
Dealing with Fractional Oxidation States
In some cases, such as superoxides, the oxidation numbers can be fractional. This arises from the inherent complexities of bonding and electron distribution in these molecules. The fractional oxidation number should not be misinterpreted as a partial charge but rather as a bookkeeping device to balance the overall charge of the compound.
Oxidation Numbers and Redox Reactions
The primary application of oxidation numbers lies in understanding and balancing redox reactions. Redox reactions involve the transfer of electrons between species, resulting in a change in the oxidation numbers of the atoms involved. Balancing redox reactions necessitates a careful tracking of these oxidation number changes.
Oxidation Numbers and Molecular Structure
The oxidation number of an atom is related, although not directly equivalent, to the charge distribution within a molecule. Understanding molecular structure, particularly bond polarity and electron density, can provide insight into the likely oxidation states of the constituent atoms.
Oxidation Numbers and Chemical Properties
The oxidation state of an element strongly influences its chemical properties. Elements in higher oxidation states often exhibit different reactivities and bonding characteristics compared to the same element in lower oxidation states. This difference in reactivity can be utilized in various chemical applications, including selective oxidation and reduction reactions.
Conclusion: Mastering Oxidation Numbers
Determining the oxidation number of iodine in IO₃⁻ provides a practical example of applying fundamental principles of chemical nomenclature. Understanding oxidation numbers is crucial for mastering redox chemistry, balancing equations, and predicting the reactivity of chemical compounds. While the basic calculation for simple ions like IO₃⁻ is straightforward, the underlying principles extend to complex scenarios involving fractional oxidation numbers and nuanced interpretations of charge distribution within molecules. A strong grasp of these principles is invaluable for any student or professional working in the field of chemistry. Further exploration into advanced concepts of oxidation states will provide a deeper understanding of chemical bonding and reactivity.
Latest Posts
Latest Posts
-
Which Of The Following Is A Function That Money Serves
Apr 01, 2025
-
The Most Abundant Compound In Most Living Things Is
Apr 01, 2025
-
How Can We Change The Polarity Of An Electromagnet
Apr 01, 2025
-
A Mother Beats Up Her Daughter Because She Was Drunk
Apr 01, 2025
-
Author Of Sare Jahan Se Acha
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Oxidation Number Of I In Io3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
