Osmosis Is Best Defined As The Movement Of
News Leon
Mar 18, 2025 · 6 min read
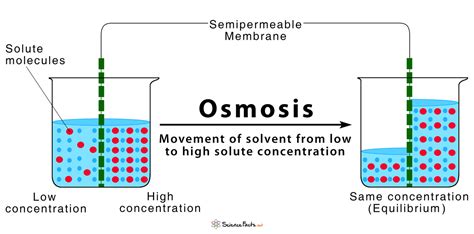
Table of Contents
Osmosis: The Movement of Water Across a Selectively Permeable Membrane
Osmosis is a fundamental process in biology, crucial for the survival and function of all living organisms. It's a type of passive transport, meaning it doesn't require energy input from the cell. But what exactly is osmosis? In its simplest definition, osmosis is the movement of water molecules across a selectively permeable membrane from a region of higher water concentration to a region of lower water concentration. This movement continues until equilibrium is reached, or until a counteracting force prevents further movement. Understanding osmosis requires delving into several key concepts, including selectively permeable membranes, water potential, and the impact of osmosis on living cells.
Understanding Selectively Permeable Membranes
The heart of osmosis lies in the selectively permeable membrane. This isn't just any barrier; it's a specialized structure that allows certain substances to pass through while restricting others. Think of it like a highly selective bouncer at an exclusive club. In biological systems, the most common selectively permeable membrane is the cell membrane, also known as the plasma membrane. This membrane is composed primarily of a phospholipid bilayer, with embedded proteins that play various roles, including transport of molecules.
The phospholipid bilayer itself is largely impermeable to water. However, water molecules are small enough to pass through tiny gaps between the phospholipids, or through specialized protein channels called aquaporins. Aquaporins dramatically increase the rate at which water can cross the membrane. This selectivity ensures that water moves across the membrane, while larger molecules or ions are largely excluded, maintaining the cell's internal environment.
Water Potential: The Driving Force Behind Osmosis
To truly grasp osmosis, understanding water potential is essential. Water potential is the measure of the tendency of water to move from one area to another. It's expressed in units of pressure (typically Pascals or megapascals). Water potential is affected by several factors:
-
Pressure Potential: This refers to the physical pressure on the water. Positive pressure potential pushes water out, while negative pressure potential (tension) pulls water in. Think of a balloon filled with water; the pressure inside is positive.
-
Solute Potential: This reflects the concentration of solutes in the water. A higher concentration of solutes lowers the water potential because the solutes bind to water molecules, reducing their availability to move. Pure water has a solute potential of zero.
The overall water potential (Ψ) is the sum of pressure potential (Ψ<sub>p</sub>) and solute potential (Ψ<sub>s</sub>): Ψ = Ψ<sub>p</sub> + Ψ<sub>s</sub>. Water will always move from an area of higher water potential to an area of lower water potential.
Osmosis in Action: Hypotonic, Isotonic, and Hypertonic Solutions
When we discuss osmosis, it's often in the context of comparing the water potential of two solutions separated by a selectively permeable membrane. Three key terms are crucial here:
-
Hypotonic Solution: A solution with a higher water potential (lower solute concentration) than the solution it's being compared to. Water will move into the cell from a hypotonic solution. This can cause the cell to swell and potentially burst (lyse) in animal cells, whereas plant cells are protected by their cell wall, resulting in turgor pressure.
-
Isotonic Solution: A solution with the same water potential as the solution it's being compared to. There's no net movement of water across the membrane; the water moves equally in both directions.
-
Hypertonic Solution: A solution with a lower water potential (higher solute concentration) than the solution it's being compared to. Water will move out of the cell into the hypertonic solution. This can cause the cell to shrink (crenate) in animal cells and plasmolyze in plant cells.
The Importance of Osmosis in Biological Systems
Osmosis plays a critical role in numerous biological processes:
-
Plant Cell Turgor Pressure: Plants rely on osmosis to maintain turgor pressure, the pressure of the cell contents against the cell wall. This pressure gives plants their rigidity and upright structure. Wilting occurs when plants lose water and turgor pressure decreases.
-
Water Uptake by Roots: Osmosis is the primary mechanism by which plants absorb water from the soil through their roots. The root cells have a lower water potential than the surrounding soil water, drawing water into the plant.
-
Nutrient Absorption: Osmosis facilitates the absorption of nutrients from the environment. The movement of water into root cells also carries dissolved nutrients with it.
-
Animal Cell Function: Osmosis is crucial for maintaining the proper balance of water and electrolytes within animal cells. Maintaining this balance is essential for cell function and overall health.
-
Kidney Function: The kidneys use osmosis to regulate the concentration of water and waste products in the blood. They selectively reabsorb water, preventing excessive water loss in urine.
-
Cell Signaling: The movement of water across membranes can play a role in cell signaling, influencing various cellular processes.
Osmosis and Reverse Osmosis
While osmosis describes the natural movement of water across a membrane, reverse osmosis is a process that uses external pressure to force water to move against its concentration gradient, from an area of lower water potential to an area of higher water potential. This technique is widely used for water purification, desalination (removing salt from seawater), and other applications.
Factors Affecting Osmotic Pressure
Several factors can influence the rate of osmosis:
-
Temperature: Higher temperatures generally increase the rate of osmosis because water molecules move faster.
-
Concentration Gradient: A steeper concentration gradient (a larger difference in water potential between the two solutions) leads to a faster rate of osmosis.
-
Membrane Permeability: The permeability of the membrane to water affects the rate of osmosis. Membranes with more aquaporins allow for faster water movement.
-
Surface Area: A larger membrane surface area allows for a faster rate of osmosis.
Osmosis and Disease
Disruptions to normal osmotic balance can have significant health consequences. For example, dehydration occurs when the body loses too much water, leading to an imbalance in electrolyte concentrations and cell function. Certain medical conditions, such as kidney failure, can also disrupt osmotic balance, requiring medical intervention.
Osmosis in Everyday Life
Osmosis isn't just a laboratory phenomenon; it's happening all around us. Examples include:
-
Food Preservation: Preserving food using salt or sugar relies on osmosis. The high solute concentration draws water out of microorganisms, inhibiting their growth and preventing spoilage.
-
Water Absorption by Paper Towels: When you use a paper towel to clean up a spill, osmosis helps to absorb the liquid.
Conclusion: The Universal Significance of Osmosis
Osmosis, the seemingly simple movement of water across a membrane, is a process of immense biological significance. From maintaining cell turgor in plants to regulating blood pressure in animals, osmosis underpins the very survival of living organisms. Understanding the principles of osmosis is crucial for grasping a wide range of biological processes and their applications in various fields. The interplay between water potential, selectively permeable membranes, and solute concentration dictates the direction and rate of water movement, impacting everything from plant growth to human health. Further exploration of osmosis will continue to reveal its multifaceted roles in the natural world and its potential for future applications.
Latest Posts
Latest Posts
-
How Many Feet Is 1 2 Miles
Mar 18, 2025
-
How Many Valence Electrons Does Mn Have
Mar 18, 2025
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Osmosis Is Best Defined As The Movement Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
