Number Of Valence Electrons In Ca
News Leon
Apr 06, 2025 · 6 min read
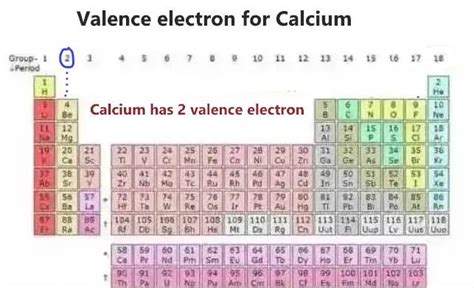
Table of Contents
Unveiling the Valence Electrons of Calcium: A Deep Dive into Atomic Structure and Chemical Behavior
Calcium (Ca), a vital element for life and a cornerstone of numerous industrial applications, holds a fascinating position in the periodic table. Understanding its electronic structure, specifically the number of valence electrons, is crucial to comprehending its chemical properties and reactivity. This comprehensive article delves into the intricacies of calcium's atomic structure, exploring its valence electrons, their significance in bonding, and their implications in various chemical and biological contexts.
What are Valence Electrons?
Before focusing specifically on calcium, let's establish a fundamental understanding of valence electrons. These are the electrons located in the outermost shell, or energy level, of an atom. They are the electrons most loosely bound to the nucleus and, therefore, participate directly in chemical bonding. The number of valence electrons determines an element's chemical reactivity and the types of bonds it can form. Atoms strive for stability, often achieving this by gaining, losing, or sharing valence electrons to attain a full outermost shell, a configuration often referred to as a noble gas configuration.
Determining the Number of Valence Electrons in Calcium
Calcium, with its atomic number of 20, possesses 20 electrons. To determine the number of valence electrons, we need to examine its electron configuration. The electron configuration of calcium is 1s²2s²2p⁶3s²3p⁶4s².
-
Understanding Electron Configurations: The electron configuration describes the arrangement of electrons in different energy levels and subshells within an atom. Each number represents a principal energy level (shell), while the letters (s, p, d, f) represent subshells within those energy levels. The superscripts indicate the number of electrons in each subshell.
-
Identifying Valence Electrons: The valence electrons are found in the outermost occupied shell. In calcium's configuration, the outermost shell is the fourth energy level (n=4), which contains two electrons in the 4s subshell. Therefore, calcium has two valence electrons.
The Significance of Calcium's Two Valence Electrons
The presence of only two valence electrons significantly influences calcium's chemical behavior. It readily loses these two electrons to achieve a stable, noble gas configuration similar to Argon (1s²2s²2p⁶3s²3p⁶). This electron loss results in the formation of a Ca²⁺ ion, a doubly charged cation.
Calcium's Reactivity: A Consequence of Valence Electrons
The ease with which calcium loses its two valence electrons explains its high reactivity, particularly with non-metals. This reactivity is evidenced by:
-
Reactions with Halogens: Calcium vigorously reacts with halogens (Group 17 elements like chlorine, bromine, and iodine) to form ionic compounds called halides. For example, the reaction with chlorine produces calcium chloride (CaCl₂), where calcium loses two electrons to form Ca²⁺ and chlorine gains one electron each to form two Cl⁻ ions. The electrostatic attraction between the oppositely charged ions forms the ionic bond.
-
Reactions with Oxygen: Calcium reacts readily with oxygen (O₂) to form calcium oxide (CaO). In this reaction, calcium loses its two valence electrons to oxygen atoms, which gain two electrons each to form O²⁻ ions.
-
Reactions with Water: Calcium reacts slowly with water to produce calcium hydroxide (Ca(OH)₂) and hydrogen gas (H₂). This reaction is slower than its reactions with halogens and oxygen.
Calcium's Role in Biological Systems: A Valence Electron Perspective
Calcium's two valence electrons play a critical role in its essential biological functions. Its ionic form, Ca²⁺, is vital for:
-
Bone and Tooth Formation: Calcium ions are major components of bone and tooth mineral, providing structural support and strength.
-
Muscle Contraction: Calcium ions are crucial in regulating muscle contraction and relaxation. The release and binding of Ca²⁺ ions trigger the interactions between muscle proteins, leading to muscle movement.
-
Nerve Impulse Transmission: Calcium ions play a significant role in neurotransmission, facilitating the release of neurotransmitters at synapses.
-
Blood Clotting: Calcium ions are essential cofactors in the complex cascade of reactions involved in blood clotting.
-
Enzyme Activity: Many enzymes require calcium ions as cofactors for their optimal activity. These enzymes are involved in a wide range of metabolic processes.
Calcium's Position in the Periodic Table and its Valence Electrons
Calcium's position in Group 2 (alkaline earth metals) of the periodic table directly reflects its two valence electrons. Group 2 elements are characterized by having two valence electrons, leading to similar chemical properties. They are all relatively reactive metals, readily losing their two valence electrons to form +2 ions. This similarity in electronic structure and chemical behavior is a fundamental principle underlying the organization of the periodic table.
Comparing Calcium's Reactivity with other Group 2 Elements
While all Group 2 elements have two valence electrons, their reactivity varies slightly. This variation is due to factors like atomic size and ionization energy. Calcium's reactivity is relatively high among the Group 2 elements, but less reactive than the more electropositive alkali metals (Group 1). The increasing atomic size down Group 2 leads to a slight decrease in ionization energy, meaning that it becomes slightly easier to remove the valence electrons as you go down the group. However, other factors, such as shielding effects, also play a role in determining overall reactivity.
Calcium's Compounds and their Applications: A Valence Electron Perspective
The chemical behavior dictated by its two valence electrons results in the formation of a wide range of calcium compounds with diverse applications. Here are a few examples:
-
Calcium Carbonate (CaCO₃): A major component of limestone, marble, and chalk, it's widely used in construction materials, as a filler in paper and plastics, and in antacids.
-
Calcium Oxide (CaO): Also known as quicklime, it's used in the production of cement, steel, and glass, as well as a soil amendment in agriculture.
-
Calcium Sulfate (CaSO₄): Found as gypsum, it’s used in plaster, drywall, and cement.
-
Calcium Chloride (CaCl₂): Used as a de-icing agent, desiccant, and in various industrial processes.
The properties of these compounds are directly linked to the ionic nature of the Ca²⁺ ion, a consequence of its two valence electrons.
Conclusion: The Central Role of Valence Electrons in Understanding Calcium
The number of valence electrons in an element is paramount in determining its chemical properties and reactivity. Calcium, with its two valence electrons, readily loses them to form a stable Ca²⁺ ion, influencing its interactions with other elements and its significant role in various chemical and biological systems. Its reactivity, its formation of ionic compounds, and its biological importance are all direct consequences of its electronic structure and the behavior of its two valence electrons. Understanding this fundamental aspect of calcium’s atomic structure allows us to appreciate its diverse applications and crucial role in the world around us. Further exploration of calcium's chemistry reveals the intricate interplay between its electronic structure, its reactivity, and its many essential functions in nature and technology.
Latest Posts
Latest Posts
-
Is Air An Element Compound Homogeneous Or Heterogeneous
Apr 08, 2025
-
Osmosis Is A Type Of Active Transport
Apr 08, 2025
-
What Is The Major Product Of This Reaction
Apr 08, 2025
-
Which Of The Following Is An Example Of Artificial Selection
Apr 08, 2025
-
How To Write A Matrix In Python
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Ca . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.