Number Of Valence Electrons In Aluminium
News Leon
Mar 18, 2025 · 6 min read
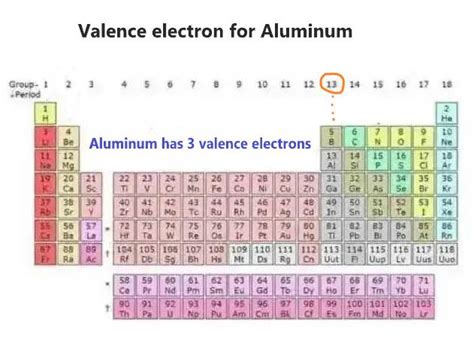
Table of Contents
Delving Deep into Aluminum: Unveiling the Secrets of its Valence Electrons
Aluminum, a ubiquitous metal found in everything from soda cans to aircraft parts, holds a fascinating position in the periodic table. Understanding its properties, especially its electronic configuration, is crucial to appreciating its widespread applications. This comprehensive article delves deep into the number of valence electrons in aluminum and explores the implications this has on its chemical behavior and reactivity.
What are Valence Electrons?
Before we dive into the specifics of aluminum, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the primary players in chemical bonding, determining an element's reactivity and the types of chemical bonds it can form (ionic, covalent, or metallic). They are the key to understanding an element's chemical behavior. The number of valence electrons dictates how many bonds an atom can form and influences its overall properties.
Determining the Number of Valence Electrons in Aluminum
Aluminum (Al) has an atomic number of 13, meaning it possesses 13 protons and, in a neutral atom, 13 electrons. To determine the number of valence electrons, we need to understand its electronic configuration.
Electronic Configuration of Aluminum
The electronic configuration of aluminum is 1s²2s²2p⁶3s²3p¹. This notation describes how electrons are distributed across different energy levels and sublevels within the atom.
- 1s²: Two electrons occupy the first energy level (n=1) in the s sublevel.
- 2s²: Two electrons occupy the second energy level (n=2) in the s sublevel.
- 2p⁶: Six electrons occupy the second energy level (n=2) in the p sublevel.
- 3s²: Two electrons occupy the third energy level (n=3) in the s sublevel.
- 3p¹: One electron occupies the third energy level (n=3) in the p sublevel.
The outermost energy level for aluminum is the third energy level (n=3). This level contains a total of three electrons (two in the 3s sublevel and one in the 3p sublevel). Therefore, aluminum has three valence electrons.
Implications of Three Valence Electrons
The presence of three valence electrons profoundly influences aluminum's chemical and physical properties. These three electrons are readily available for participation in chemical bonding. This explains why aluminum is a relatively reactive metal.
Reactivity and Chemical Bonding
Aluminum's three valence electrons readily participate in chemical reactions, often losing these electrons to achieve a stable octet configuration (eight electrons in its outermost shell), mimicking the electron configuration of the noble gas neon. This tendency to lose electrons makes aluminum a good reducing agent. It readily reacts with oxidizing agents like oxygen and halogens.
Formation of Ionic Bonds:
Aluminum's tendency to lose three electrons is crucial in the formation of ionic bonds. When reacting with nonmetals like oxygen or chlorine, aluminum readily loses its three valence electrons, forming a 3+ ion (Al³⁺). These electrons are then gained by the nonmetal, resulting in the formation of an ionic compound. For example, in the formation of aluminum oxide (Al₂O₃), each aluminum atom loses three electrons to two oxygen atoms, which each gain two electrons. This electrostatic attraction between the oppositely charged ions forms the ionic bond.
Formation of Metallic Bonds:
In its elemental form, aluminum exhibits metallic bonding. Its three valence electrons are delocalized, meaning they are not bound to any particular atom but rather move freely throughout the metal lattice. This sea of delocalized electrons accounts for many of aluminum's characteristic properties, such as its excellent electrical and thermal conductivity, malleability, and ductility.
Oxidation and Corrosion
The reactivity of aluminum's valence electrons also plays a significant role in its oxidation and corrosion behavior. When exposed to air, aluminum readily reacts with oxygen to form a thin, protective layer of aluminum oxide (Al₂O₃). This oxide layer is extremely stable and acts as a barrier, preventing further oxidation and protecting the underlying aluminum from corrosion. This explains why aluminum is surprisingly resistant to corrosion, despite being a relatively reactive metal. This passive layer is crucial for its use in various applications exposed to harsh environments.
Aluminum's Applications: A Testament to its Valence Electrons
The unique properties of aluminum, directly attributable to its three valence electrons, lead to a vast array of applications across diverse industries:
- Packaging: Aluminum foil and cans are widely used due to their lightweight, malleable nature, and resistance to corrosion.
- Transportation: Aluminum alloys are used extensively in the automotive and aerospace industries due to their high strength-to-weight ratio.
- Construction: Aluminum's corrosion resistance makes it suitable for building materials, such as window frames, doors, and siding.
- Electrical applications: Aluminum's excellent electrical conductivity makes it a preferred material for electrical wiring and transmission lines.
- Consumer electronics: Aluminum is used in the manufacture of various electronic devices due to its lightweight and ability to be easily shaped.
Comparing Aluminum to Other Elements
Understanding the number of valence electrons in aluminum allows us to compare and contrast its properties with other elements. For example:
- Magnesium (Mg): Magnesium has two valence electrons and is less reactive than aluminum.
- Silicon (Si): Silicon has four valence electrons and forms covalent bonds, resulting in very different properties compared to aluminum.
- Sodium (Na): Sodium, like aluminum, has a low number of valence electrons (one), but its reactivity is higher due to only one electron needing to be lost to achieve a stable octet.
- Boron (B): Boron has three valence electrons just like aluminum. However, its properties differ significantly due to its smaller size and different electronic configuration.
Comparing these elements highlights how the number of valence electrons directly impacts an element's behavior and properties.
Conclusion: The Significance of Aluminum's Valence Electrons
The presence of three valence electrons fundamentally defines aluminum's chemical and physical properties. This seemingly simple fact underpins aluminum's widespread use in countless applications. Its reactivity, ability to form both ionic and metallic bonds, and the formation of a protective oxide layer are all direct consequences of its electronic configuration. A comprehensive understanding of valence electrons is not only crucial for comprehending aluminum's behavior but also for predicting and designing new materials and technologies utilizing this versatile metal. Further exploration into the intricacies of its electronic structure reveals a deeper appreciation for the remarkable properties of this ubiquitous element. From everyday household items to high-tech applications, aluminum's story is intrinsically linked to the behavior of its three valence electrons. The study of valence electrons serves as a foundational principle in chemistry, providing a crucial framework for understanding the diverse properties of elements across the periodic table.
Latest Posts
Latest Posts
-
How Many Feet Is 1 2 Miles
Mar 18, 2025
-
How Many Valence Electrons Does Mn Have
Mar 18, 2025
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Aluminium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
