No Two Different Elements Will Have The
News Leon
Mar 30, 2025 · 5 min read
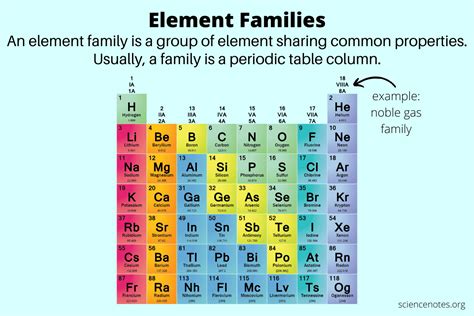
Table of Contents
No Two Different Elements Will Have the Same Atomic Number: A Deep Dive into Atomic Structure and its Implications
The statement "no two different elements will have the same atomic number" is a fundamental principle in chemistry and physics. Understanding this principle requires a deep dive into the structure of atoms, the building blocks of all matter. This article will explore this principle in detail, examining its implications for the periodic table, chemical properties, and our understanding of the universe.
Understanding Atomic Number
The atomic number of an element is defined as the number of protons found in the nucleus of an atom of that element. Protons are positively charged subatomic particles and are crucial in determining the identity of an element. Unlike neutrons (neutral subatomic particles) and electrons (negatively charged subatomic particles), the number of protons is unique to each element.
The Significance of Protons
Protons, along with neutrons, form the atom's nucleus, which constitutes the vast majority of its mass. Electrons, on the other hand, orbit the nucleus in shells or energy levels. The number of electrons in a neutral atom is equal to the number of protons, ensuring a balanced electrical charge. However, it's the number of protons, specifically, that dictates an element's identity and its place on the periodic table.
Isotopes and Neutron Variation
It's important to note that atoms of the same element can have different numbers of neutrons. These variations are called isotopes. For example, carbon-12 and carbon-14 are both isotopes of carbon. They both have six protons (defining them as carbon), but carbon-12 has six neutrons, while carbon-14 has eight. While isotopes have different masses, they share the same atomic number and thus the same fundamental chemical properties. This is because the chemical behavior of an atom is primarily determined by its electron configuration, which is directly linked to the number of protons.
The Periodic Table and Atomic Number
The periodic table of elements is organized by atomic number. Elements are arranged in rows (periods) and columns (groups) based on their atomic number and recurring chemical properties. The arrangement reflects the periodic nature of electron shell filling, leading to the observed patterns of reactivity and other chemical characteristics.
Mendeleev's Vision and Atomic Number
Dmitri Mendeleev, the creator of the periodic table, didn't initially know about atomic numbers. He arranged elements based on their atomic weight and observed similarities in their properties. Later, it was discovered that the ordering by atomic weight mostly aligned with the ordering by atomic number, providing a strong theoretical basis for the table's organization.
Atomic Number as the Primary Organizing Principle
The atomic number is the ultimate organizing principle of the periodic table. It ensures that elements with similar electronic configurations and therefore similar chemical properties are grouped together. This is crucial for predicting and understanding chemical reactions and the behavior of different substances.
The Implications of Unique Atomic Numbers
The fact that no two different elements share the same atomic number has profound implications across various scientific fields.
Distinguishing Elements
The unique atomic number allows us to unambiguously distinguish one element from another. This is critical for identifying the composition of substances, analyzing materials, and understanding chemical reactions. Analytical techniques like mass spectrometry rely heavily on this principle to accurately determine the elemental composition of samples.
Predicting Chemical Properties
The atomic number plays a vital role in predicting the chemical properties of an element. The number of protons determines the number of electrons, which in turn dictates the electron configuration. The electron configuration determines how an atom will interact with other atoms, influencing its reactivity, bonding behavior, and overall chemical properties.
Understanding Nuclear Reactions
In nuclear physics, the atomic number is essential for understanding nuclear reactions. Nuclear reactions involve changes in the nucleus of an atom, often leading to a change in the atomic number, thus transforming one element into another. This is the basis of nuclear fission and fusion, processes that have vast implications for energy production and other technological applications.
Astrophysics and Stellar Nucleosynthesis
In astrophysics, the atomic number is key to understanding the formation of elements in stars. Stellar nucleosynthesis involves the creation of heavier elements from lighter ones through nuclear fusion reactions within stars. The atomic number tracks the transformation of elements during this process, helping us to understand the origin of the elements in the universe.
Beyond the Basics: Isotopes and Their Applications
While the atomic number defines the element, the number of neutrons can vary, leading to isotopes. Isotopes are crucial in several applications:
Radioactive Isotopes and Medical Imaging
Certain isotopes are radioactive, meaning their nuclei are unstable and decay over time, emitting radiation. These radioactive isotopes have many applications in medicine, such as in medical imaging techniques like PET (positron emission tomography) and SPECT (single-photon emission computed tomography). These techniques use radioactive tracers to visualize and diagnose various medical conditions.
Radioactive Dating
Radioactive isotopes also play a significant role in radioactive dating, a method used to determine the age of geological formations and artifacts. By measuring the ratio of parent isotopes to daughter isotopes, scientists can estimate the time elapsed since the sample's formation. Carbon-14 dating, for example, is used to date organic materials up to around 50,000 years old.
Industrial Applications of Isotopes
Isotopes also find various applications in industry. They are used in tracer studies to track the movement of materials in industrial processes and for gauging the thickness of materials. Stable isotopes are employed in various analytical techniques, providing valuable information about the composition and origin of substances.
Conclusion: The Cornerstone of Chemistry
The principle that no two different elements will have the same atomic number is a cornerstone of modern chemistry and physics. This seemingly simple statement underpins our understanding of the periodic table, chemical properties, nuclear reactions, and the composition of matter in the universe. From medical imaging to radioactive dating and the study of stellar nucleosynthesis, the atomic number plays a crucial role in many scientific fields, highlighting its fundamental importance in our understanding of the world around us. Continued research into atomic structure and its implications promises to unveil further insights into the complexity and beauty of the natural world. The implications of unique atomic numbers are far-reaching, impacting our technologies, medical advancements, and fundamental understanding of the universe. The significance of this principle cannot be overstated. It’s a testament to the power of fundamental scientific principles in driving innovation and understanding.
Latest Posts
Latest Posts
-
State Any Two Effects Of Force
Apr 01, 2025
-
How Many Electrons Does Sodium Have In Its Outer Shell
Apr 01, 2025
-
Dense Irregular Connective Tissue Will Be Found In The
Apr 01, 2025
-
Which Of The Following Organisms Can Fix Nitrogen
Apr 01, 2025
-
Which Of The Following Is Correct About Viruses
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about No Two Different Elements Will Have The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
