Net Ionic Equation Of Hcl And Naoh
News Leon
Mar 16, 2025 · 6 min read
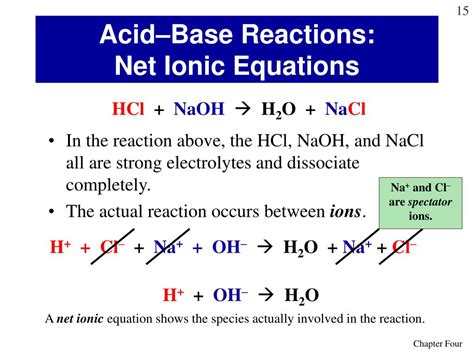
Table of Contents
Net Ionic Equation of HCl and NaOH: A Deep Dive into Acid-Base Reactions
The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is a classic example of a strong acid-strong base neutralization reaction. Understanding its net ionic equation is crucial for grasping fundamental concepts in chemistry, particularly stoichiometry and acid-base chemistry. This comprehensive guide will explore the reaction in detail, explaining the process of deriving the net ionic equation, its significance, and its applications.
Understanding the Reactants: HCl and NaOH
Before diving into the reaction, let's examine the individual reactants:
Hydrochloric Acid (HCl)
Hydrochloric acid is a strong acid, meaning it completely dissociates in water, releasing hydrogen ions (H⁺) and chloride ions (Cl⁻):
HCl(aq) → H⁺(aq) + Cl⁻(aq)
This complete dissociation is key to understanding the ionic equation. The presence of freely moving ions in solution is what allows for the reaction with NaOH.
Sodium Hydroxide (NaOH)
Sodium hydroxide is a strong base, also completely dissociating in water into sodium ions (Na⁺) and hydroxide ions (OH⁻):
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
Similar to HCl, the complete dissociation of NaOH is essential for the reaction to proceed effectively. The hydroxide ions are crucial for neutralizing the hydrogen ions from the acid.
The Complete Ionic Equation
When HCl and NaOH react, the hydrogen ions (H⁺) from the acid combine with the hydroxide ions (OH⁻) from the base to form water (H₂O). The sodium ions (Na⁺) and chloride ions (Cl⁻) remain in solution as spectator ions. This leads to the complete ionic equation:
H⁺(aq) + Cl⁻(aq) + Na⁺(aq) + OH⁻(aq) → Na⁺(aq) + Cl⁻(aq) + H₂O(l)
This equation shows all the ions present in the solution before and after the reaction. Notice that some ions appear on both sides of the equation – these are the spectator ions.
Identifying and Removing Spectator Ions
Spectator ions are ions that do not participate directly in the chemical reaction. They remain unchanged throughout the process. In this case, Na⁺ and Cl⁻ are spectator ions. They are present before and after the reaction, simply floating around in the solution. To obtain the net ionic equation, we remove these spectator ions from the complete ionic equation.
Deriving the Net Ionic Equation
By eliminating the spectator ions (Na⁺ and Cl⁻) from the complete ionic equation, we arrive at the net ionic equation:
H⁺(aq) + OH⁻(aq) → H₂O(l)
This equation represents the essence of the reaction: the combination of hydrogen ions and hydroxide ions to form water. It is significantly simpler than the complete ionic equation, yet it conveys the crucial chemical change that occurs. This simplicity is a great advantage when analyzing and understanding chemical reactions.
Significance of the Net Ionic Equation
The net ionic equation holds significant importance for several reasons:
-
Simplicity and Clarity: It provides a concise representation of the chemical reaction, focusing solely on the species that actively participate in the reaction. This enhances understanding and simplifies calculations.
-
Stoichiometry: The net ionic equation is essential for stoichiometric calculations. It provides the correct mole ratios between the reacting species, allowing for accurate predictions of product quantities.
-
Predicting Reactions: The net ionic equation can help predict the outcome of similar reactions involving strong acids and strong bases. As long as the acid and base completely dissociate, the net ionic equation will remain consistent.
-
Understanding Acid-Base Neutralization: The net ionic equation clearly demonstrates the fundamental principle of acid-base neutralization: the combination of H⁺ and OH⁻ to form water, leading to a neutral solution (pH 7).
Applications of the Net Ionic Equation
The concepts learned from the HCl and NaOH reaction, and specifically the net ionic equation, have broad applications in various fields:
-
Titration: The net ionic equation is crucial in titrations, where the concentration of an unknown acid or base is determined by reacting it with a solution of known concentration. Understanding the stoichiometry from the net ionic equation allows for accurate calculations.
-
Environmental Chemistry: Acid-base reactions are prevalent in environmental systems. Understanding net ionic equations helps in analyzing and managing water quality, particularly in scenarios involving acid rain or industrial wastewater.
-
Biological Systems: Many biological processes involve acid-base reactions. The principles learned from the net ionic equation are applicable in understanding biochemical processes and maintaining pH balance in living organisms.
Beyond Strong Acids and Bases: Weak Acids and Bases
While the HCl and NaOH reaction provides a clear example, it's crucial to understand that not all acid-base reactions result in such a straightforward net ionic equation. Reactions involving weak acids or weak bases are more complex.
Weak acids and weak bases do not completely dissociate in water. They exist in equilibrium between the undissociated molecule and its ions. Therefore, the complete and net ionic equations for reactions involving weak acids or bases will include the undissociated acid or base molecule.
For instance, consider the reaction between acetic acid (CH₃COOH, a weak acid) and NaOH:
Complete Ionic Equation: CH₃COOH(aq) + Na⁺(aq) + OH⁻(aq) → CH₃COO⁻(aq) + Na⁺(aq) + H₂O(l)
Net Ionic Equation: CH₃COOH(aq) + OH⁻(aq) → CH₃COO⁻(aq) + H₂O(l)
Notice that the acetic acid molecule remains in the net ionic equation because it doesn't fully dissociate. The equilibrium nature of the reaction needs to be considered for a complete understanding.
Further Exploration: Solubility and Precipitation Reactions
The principles of net ionic equations extend beyond acid-base reactions. They are also essential for understanding precipitation reactions, where insoluble salts are formed. These reactions often involve spectator ions, and the net ionic equation highlights the formation of the precipitate.
For example, consider the reaction between silver nitrate (AgNO₃) and sodium chloride (NaCl):
Complete Ionic Equation: Ag⁺(aq) + NO₃⁻(aq) + Na⁺(aq) + Cl⁻(aq) → AgCl(s) + Na⁺(aq) + NO₃⁻(aq)
Net Ionic Equation: Ag⁺(aq) + Cl⁻(aq) → AgCl(s)
Here, the net ionic equation shows the formation of the insoluble silver chloride (AgCl) precipitate. The sodium and nitrate ions are spectator ions.
Conclusion
The net ionic equation for the reaction between HCl and NaOH, H⁺(aq) + OH⁻(aq) → H₂O(l), serves as a fundamental example of acid-base neutralization. Understanding how to derive this equation, its significance, and its applications extends to a broader comprehension of acid-base chemistry, stoichiometry, and solution chemistry. The concepts discussed, including the distinction between strong and weak electrolytes and the identification of spectator ions, are crucial for solving various chemical problems and understanding complex chemical phenomena. By mastering these concepts, you build a solid foundation for more advanced studies in chemistry and related fields. Remember that while the HCl and NaOH example is straightforward, the principles apply broadly to a wide range of reactions, including those involving weak acids and bases and precipitation reactions. This deep dive into the net ionic equation demonstrates its vital role in chemical analysis and prediction.
Latest Posts
Latest Posts
-
What Is A Non Permanent Magnet
Mar 16, 2025
-
3 Cards Same From 52 Probability
Mar 16, 2025
-
Which Cell Organelle Is Found Only In Plant Cell
Mar 16, 2025
-
The Summer Of The White Horse
Mar 16, 2025
-
How To Separate Water And Gasoline
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Net Ionic Equation Of Hcl And Naoh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
