Net Ionic Equation Hcl + Naoh
News Leon
Mar 21, 2025 · 5 min read
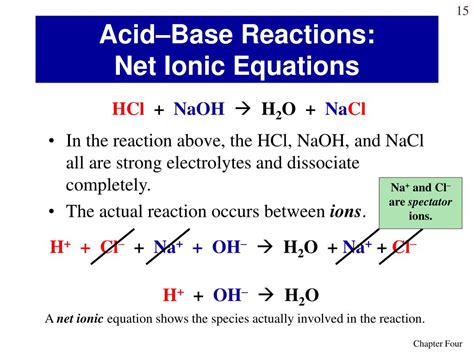
Table of Contents
Net Ionic Equation: HCl + NaOH – A Deep Dive into Acid-Base Reactions
The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is a classic example of a strong acid-strong base neutralization reaction. Understanding this reaction, especially its net ionic equation, is fundamental to grasping the concepts of acid-base chemistry and stoichiometry. This article provides a comprehensive exploration of the HCl + NaOH reaction, delving into its balanced molecular equation, total ionic equation, and ultimately, the crucial net ionic equation. We will also explore the spectator ions, the concepts behind neutralization reactions, and practical applications.
Understanding the Reactants: HCl and NaOH
Before diving into the reaction itself, let's briefly examine the properties of the reactants: hydrochloric acid (HCl) and sodium hydroxide (NaOH).
Hydrochloric Acid (HCl)
Hydrochloric acid is a strong acid, meaning it completely dissociates into its ions in aqueous solution. This dissociation is represented as follows:
HCl(aq) → H⁺(aq) + Cl⁻(aq)
The hydrogen ion (H⁺) is responsible for the acidic properties of HCl. It readily donates a proton (H⁺) to other molecules or ions, a characteristic defining an acid according to the Brønsted-Lowry theory.
Sodium Hydroxide (NaOH)
Sodium hydroxide is a strong base, completely dissociating in aqueous solution to form sodium ions (Na⁺) and hydroxide ions (OH⁻). Its dissociation is:
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
The hydroxide ion (OH⁻) is responsible for the basic properties of NaOH. It readily accepts a proton (H⁺), a defining characteristic of a base according to the Brønsted-Lowry theory. It's crucial to remember that the hydroxide ion's ability to accept protons is the key to its reactivity.
The Balanced Molecular Equation
When hydrochloric acid and sodium hydroxide react, they undergo a neutralization reaction, forming water and a salt. The balanced molecular equation represents this reaction:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
This equation shows that one mole of HCl reacts with one mole of NaOH to produce one mole of sodium chloride (NaCl) and one mole of water (H₂O). The (aq) indicates that the substance is dissolved in water (aqueous solution), and (l) denotes a liquid. This balanced equation shows the stoichiometric ratio between the reactants and products.
The Total Ionic Equation
To understand the net ionic equation, we first need to write the total ionic equation. This equation shows all the ions present in the solution before and after the reaction. Since HCl and NaOH are strong electrolytes, they completely dissociate into their respective ions. Thus, the total ionic equation is:
H⁺(aq) + Cl⁻(aq) + Na⁺(aq) + OH⁻(aq) → Na⁺(aq) + Cl⁻(aq) + H₂O(l)
The Net Ionic Equation: The Heart of the Reaction
The net ionic equation represents the actual chemical change occurring during the reaction. It is derived from the total ionic equation by removing the spectator ions. Spectator ions are ions that appear on both sides of the total ionic equation and do not participate directly in the reaction. In this case, Na⁺ and Cl⁻ are spectator ions. Removing them leaves us with the net ionic equation:
H⁺(aq) + OH⁻(aq) → H₂O(l)
This simple yet profound equation represents the essence of the acid-base neutralization reaction. It shows that the hydrogen ion from the acid reacts with the hydroxide ion from the base to form water. This is the fundamental process that defines acid-base neutralization. The formation of water is the driving force behind this reaction.
Significance of the Net Ionic Equation
The net ionic equation is crucial for several reasons:
- Simplicity: It simplifies the representation of the reaction, focusing only on the species that undergo chemical change.
- Understanding the reaction mechanism: It highlights the actual chemical process occurring at the molecular level. It directly shows the proton transfer from the acid to the base.
- Predicting reactions: It allows for prediction of similar reactions involving strong acids and strong bases. Any strong acid reacting with a strong base will have the same net ionic equation.
- Stoichiometric calculations: While the balanced molecular equation is used for calculating overall amounts of reactants and products, the net ionic equation focuses on the specific reacting species for precise stoichiometric analysis concerning the core reaction.
Applications of the HCl + NaOH Reaction
The neutralization reaction between HCl and NaOH has various applications:
- Titrations: This reaction is fundamental to acid-base titrations, a quantitative analytical technique used to determine the concentration of an unknown acid or base. The reaction's stoichiometry is crucial for accurate calculations.
- pH Control: The reaction is used to adjust the pH of solutions, vital in many chemical processes and industrial applications.
- Wastewater Treatment: Neutralization reactions are essential for treating wastewater containing acidic or basic components to make them environmentally safe.
- Chemical Synthesis: Controlled acid-base neutralization reactions are often used in the synthesis of various chemical compounds.
Beyond the Basics: Variations and Considerations
While this article focuses on the reaction of a strong acid and a strong base, it's important to consider other scenarios.
- Weak Acids and Bases: Reactions involving weak acids and bases do not completely dissociate, leading to different total and net ionic equations. The equilibrium expressions become important in these cases.
- Polyprotic Acids: Acids donating more than one proton (e.g., sulfuric acid, H₂SO₄) will have more complex net ionic equations, with each proton transfer represented individually.
- Temperature Dependence: While less pronounced for strong acid-strong base reactions, the equilibrium constant (K) can be temperature-dependent.
Conclusion: A Fundamental Reaction in Chemistry
The reaction between HCl and NaOH, with its simplified net ionic equation of H⁺(aq) + OH⁻(aq) → H₂O(l), stands as a cornerstone of acid-base chemistry. Understanding this reaction thoroughly, including its balanced molecular equation and total ionic equation, is essential for anyone studying chemistry. The principles learned here are readily transferable to understanding countless other acid-base neutralization reactions, laying a strong foundation for more advanced topics. The simplicity of the net ionic equation belies its importance in analytical chemistry, industrial processes, and environmental science. The concept of spectator ions and the underlying stoichiometry underpin many quantitative analytical techniques. Remember, the formation of water is the key driver of the reaction and the focus of the essential net ionic equation.
Latest Posts
Latest Posts
-
If A And B Are Independent Events
Mar 21, 2025
-
How Many Seconds Is In 4 Minutes
Mar 21, 2025
-
Monomers That Make Up Nucleic Acids
Mar 21, 2025
-
If An Enzyme In Solution Is Saturated With Substrate
Mar 21, 2025
-
What Class Of Lever Is A Wheelbarrow
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Net Ionic Equation Hcl + Naoh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
