If An Enzyme In Solution Is Saturated With Substrate
News Leon
Mar 21, 2025 · 7 min read
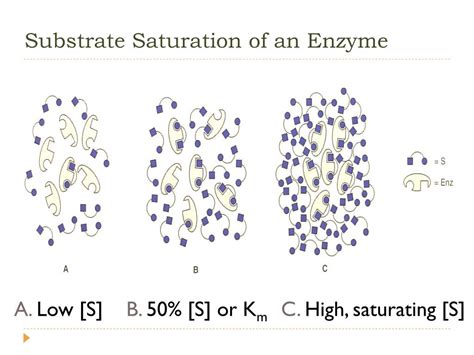
Table of Contents
If an Enzyme in Solution is Saturated with Substrate
Enzyme kinetics is a cornerstone of biochemistry, providing crucial insights into the intricate mechanisms of biological reactions. A key concept within this field is the understanding of enzyme saturation with substrate. This article will delve deeply into this phenomenon, exploring its implications for reaction rates, enzyme activity, and the overall function of enzymatic pathways.
Understanding Enzyme-Substrate Interaction
Enzymes are biological catalysts, significantly accelerating the rate of biochemical reactions without being consumed in the process. They achieve this by binding to specific molecules, known as substrates, at their active sites. This binding forms an enzyme-substrate complex (ES complex), a crucial intermediate in the catalytic process. The active site possesses a unique three-dimensional structure perfectly complementary to the substrate, ensuring high specificity and efficiency.
The interaction between the enzyme and substrate follows a lock-and-key model or, more accurately, an induced-fit model. The lock-and-key model suggests a rigid enzyme structure perfectly fitting the substrate. The induced-fit model, however, proposes that the enzyme's active site undergoes conformational changes upon substrate binding, optimizing the interaction for catalysis. This conformational change is crucial for the catalytic process.
The Michaelis-Menten Equation: A Quantitative Description
The rate of an enzyme-catalyzed reaction is not simply linearly proportional to substrate concentration. Instead, it follows a characteristic saturation curve, elegantly described by the Michaelis-Menten equation:
v = (V<sub>max</sub>[S]) / (K<sub>m</sub> + [S])
Where:
- v represents the initial reaction velocity.
- V<sub>max</sub> is the maximum reaction velocity, reached when the enzyme is saturated with substrate.
- [S] represents the substrate concentration.
- K<sub>m</sub> (the Michaelis constant) is the substrate concentration at which the reaction velocity is half of V<sub>max</sub>. It reflects the enzyme's affinity for its substrate; a lower K<sub>m</sub> indicates higher affinity.
This equation reveals the relationship between substrate concentration and reaction velocity. At low substrate concentrations, the reaction rate increases proportionally with [S]. However, as [S] increases, the rate approaches V<sub>max</sub> asymptotically. This signifies that the enzyme is becoming saturated with substrate.
Enzyme Saturation: The Plateau Effect
When an enzyme in solution is saturated with substrate, it means that all available active sites on the enzyme molecules are occupied by substrate molecules. At this point, increasing the substrate concentration further will not lead to a significant increase in the reaction rate. The reaction velocity plateaus at V<sub>max</sub>. This is because all the enzyme molecules are working at their maximum capacity; they are already processing substrate molecules as fast as they can. Adding more substrate won't speed up the process as there are no free active sites available to bind to them.
Implications of Enzyme Saturation
The saturation of an enzyme with its substrate has several significant implications:
- Maximum Reaction Rate: It defines the upper limit of the reaction rate for a given enzyme concentration. This V<sub>max</sub> is a crucial parameter used to characterize enzyme activity.
- Regulatory Control: The saturation phenomenon plays a critical role in the regulation of metabolic pathways. By controlling substrate levels, cells can fine-tune the rates of enzymatic reactions. If substrate levels exceed the saturation point, there's no further increase in product formation, preventing wasteful overproduction.
- Enzyme Inhibition Studies: Understanding enzyme saturation is vital in studying enzyme inhibition. Competitive inhibitors compete with the substrate for binding to the active site. The presence of a competitive inhibitor increases the apparent K<sub>m</sub>, requiring a higher substrate concentration to achieve half V<sub>max</sub>. Non-competitive inhibitors, however, reduce V<sub>max</sub> without affecting K<sub>m</sub>.
- Drug Development: Understanding enzyme kinetics and saturation is crucial in drug design. Many drugs act by inhibiting enzymes involved in disease processes. Knowledge of the enzyme's K<sub>m</sub> and V<sub>max</sub> values helps determine the optimal drug concentration for effective inhibition.
Factors Affecting Enzyme Saturation
Several factors influence the point at which an enzyme becomes saturated with substrate:
- Enzyme Concentration: A higher enzyme concentration leads to a higher V<sub>max</sub>, as more active sites are available to bind substrate. The saturation point will be reached at a higher substrate concentration.
- Temperature: Temperature affects enzyme activity. Optimal temperature allows the enzyme to work at its maximum rate. At very high temperatures, enzymes denature, losing their catalytic activity. At very low temperatures, the enzyme's reaction rate slows down.
- pH: Similar to temperature, pH affects enzyme structure and activity. Each enzyme has an optimal pH range for its function. Extreme pH values can denature the enzyme.
- Presence of Inhibitors or Activators: Inhibitors reduce enzyme activity, thereby reducing V<sub>max</sub> or increasing K<sub>m</sub>. Activators enhance enzyme activity and can increase V<sub>max</sub>.
- Substrate Structure and Affinity: The substrate's structure and its affinity to the enzyme greatly influence how easily it binds and how quickly the enzyme becomes saturated.
- Allosteric Regulation: Some enzymes are regulated allosterically, meaning a molecule binding to a site other than the active site can alter the enzyme's activity, affecting its ability to reach saturation.
Beyond Michaelis-Menten: More Complex Kinetics
While the Michaelis-Menten equation provides a useful model for many enzyme-catalyzed reactions, it has limitations. It assumes:
- Steady-state assumption: The concentration of the ES complex remains relatively constant during the initial phase of the reaction.
- Single substrate reaction: The enzyme only binds one substrate.
- No product inhibition: The product does not inhibit the enzyme's activity.
Many enzyme reactions deviate from these assumptions. For instance, some enzymes catalyze reactions with multiple substrates, exhibiting more complex kinetics. Others exhibit cooperative binding, where the binding of one substrate molecule influences the binding of subsequent molecules. These cases require more elaborate kinetic models to describe the reaction accurately.
Experimental Determination of V<sub>max</sub> and K<sub>m</sub>
The Michaelis-Menten parameters, V<sub>max</sub> and K<sub>m</sub>, can be experimentally determined. Common methods include:
- Direct Linear Plot: This method plots the reciprocal of the reaction velocity (1/v) against the reciprocal of the substrate concentration (1/[S]). This creates a Lineweaver-Burk plot, where the y-intercept represents 1/V<sub>max</sub> and the x-intercept represents -1/K<sub>m</sub>.
- Hanes-Woolf Plot: This method plots [S]/v against [S]. The slope of this plot is 1/V<sub>max</sub>, and the x-intercept is -K<sub>m</sub>.
- Eadie-Hofstee Plot: This method plots v/[S] against v. The slope of this plot is -1/K<sub>m</sub>, and the x-intercept is V<sub>max</sub>.
These graphical methods allow for the determination of kinetic parameters from experimental data, providing quantitative insights into enzyme activity and substrate affinity. Modern techniques also utilize sophisticated software and fitting algorithms for more precise determination of these parameters.
Conclusion: The Significance of Enzyme Saturation in Biological Systems
Enzyme saturation is a fundamental concept in biochemistry with broad implications for understanding biological processes. It highlights the relationship between substrate concentration, enzyme activity, and reaction rate. Reaching V<sub>max</sub> signifies the enzyme’s maximal capacity, influencing metabolic control and pathway regulation. Understanding enzyme saturation is crucial for interpreting experimental data, designing drugs, and comprehending the intricate mechanisms that govern biological systems. Moreover, appreciating the limitations of the Michaelis-Menten model leads to the exploration of more complex kinetic models to account for the diversity of enzyme-substrate interactions in living organisms. Continued research in enzyme kinetics continues to unveil further details on this essential aspect of biochemistry.
Latest Posts
Latest Posts
-
Why Do Stars Only Come Out At Night
Mar 22, 2025
-
What Does The Phrase Like Dissolves Like Mean
Mar 22, 2025
-
Draw 10 Water Molecules To Create A Cluster
Mar 22, 2025
-
Half Of 1 And 1 2 Teaspoons
Mar 22, 2025
-
Number Of Cells In The Interphase
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about If An Enzyme In Solution Is Saturated With Substrate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
