Moles Of Solute Per Liter Of Solution
News Leon
Mar 20, 2025 · 5 min read
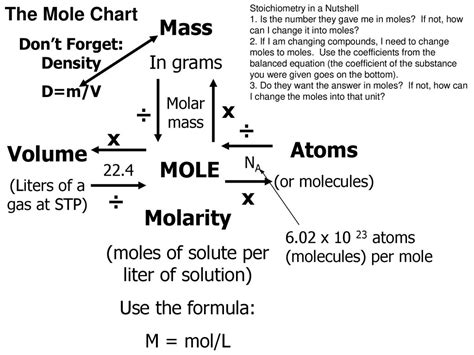
Table of Contents
Moles of Solute Per Liter of Solution: A Comprehensive Guide
Understanding the concentration of solutions is fundamental in chemistry and numerous related fields. One of the most common and crucial ways to express concentration is in terms of moles of solute per liter of solution, more formally known as molarity (M). This article delves deep into the concept of molarity, exploring its definition, calculation, applications, limitations, and related concepts. We'll also examine how molarity plays a critical role in various chemical processes and analyses.
Defining Molarity: The Heart of Solution Concentration
Molarity, denoted by the symbol M, represents the number of moles of a solute dissolved in one liter of a solution. It's a crucial measure because it directly relates the amount of solute (in moles) to the volume of the solution (in liters). This allows for precise calculations and comparisons of the concentration across different solutions. The formula for calculating molarity is elegantly simple:
Molarity (M) = Moles of solute / Liters of solution
Understanding each component of this formula is vital:
-
Moles of solute: This refers to the amount of the substance being dissolved. A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of entities (atoms, molecules, ions, etc.). The number of moles can be calculated using the molar mass of the solute and its mass (in grams).
-
Liters of solution: This is the total volume of the homogeneous mixture formed after dissolving the solute in the solvent. It's crucial to note that it's the volume of the solution, not just the volume of the solvent used.
Calculating Molarity: A Step-by-Step Approach
Let's illustrate molarity calculations with a few examples:
Example 1: Simple Molarity Calculation
Suppose we dissolve 58.44 grams of NaCl (sodium chloride, molar mass = 58.44 g/mol) in enough water to make 1.00 liter of solution. To calculate the molarity:
-
Calculate the number of moles of NaCl: Moles = mass / molar mass = 58.44 g / 58.44 g/mol = 1.00 mol
-
Determine the volume of the solution: The volume is given as 1.00 L.
-
Calculate the molarity: Molarity = Moles of solute / Liters of solution = 1.00 mol / 1.00 L = 1.00 M
Example 2: Molarity Calculation with Volume Conversion
Let's say we dissolve 20.0 grams of NaOH (sodium hydroxide, molar mass = 40.00 g/mol) in 500 milliliters (mL) of water. To calculate the molarity:
-
Convert mL to L: 500 mL * (1 L / 1000 mL) = 0.500 L
-
Calculate the number of moles of NaOH: Moles = mass / molar mass = 20.0 g / 40.00 g/mol = 0.500 mol
-
Calculate the molarity: Molarity = Moles of solute / Liters of solution = 0.500 mol / 0.500 L = 1.00 M
Example 3: Molarity Calculation with Dilution
Dilution involves reducing the concentration of a solution by adding more solvent. The number of moles of solute remains constant during dilution, but the volume of the solution increases. We can use the following equation:
M1V1 = M2V2
where:
- M1 = initial molarity
- V1 = initial volume
- M2 = final molarity
- V2 = final volume
Let's say we have 250 mL of a 2.00 M solution of HCl. We want to dilute it to 1.00 M. What volume of water should we add?
-
Rearrange the equation to solve for V2: V2 = (M1V1) / M2
-
Substitute the values: V2 = (2.00 M * 0.250 L) / 1.00 M = 0.500 L
-
Find the volume of water to add: Since the initial volume is 0.250 L, we need to add 0.500 L - 0.250 L = 0.250 L of water.
Applications of Molarity: From Labs to Industries
Molarity is indispensable across various scientific and industrial applications:
-
Titrations: Molarity is crucial in titrations, a quantitative chemical analysis technique to determine the concentration of a solution using a solution of known concentration.
-
Stoichiometric Calculations: Molarity allows precise stoichiometric calculations, enabling accurate predictions of reaction yields and reactant requirements.
-
Pharmaceutical Preparations: Precise concentrations are vital in pharmaceutical formulations, ensuring accurate drug dosages.
-
Environmental Monitoring: Molarity helps analyze pollutants in water and air samples, facilitating environmental protection efforts.
-
Food and Beverage Industry: Molarity plays a role in controlling the concentration of ingredients in various food and beverage products.
-
Industrial Processes: Many industrial processes rely on solutions of precise concentrations, making molarity an essential tool for process control and optimization.
Limitations of Molarity: Considering Temperature and Volume Changes
While molarity is a widely used and practical measure, it does have some limitations:
-
Temperature Dependence: Molarity is temperature-dependent because the volume of a solution changes with temperature. Therefore, molarity values should ideally be reported with the corresponding temperature.
-
Volume Changes: Molarity doesn't account for changes in the volume of a solution during chemical reactions or due to other factors.
Related Concepts: Expanding the Understanding of Concentration
Several other concentration units are closely related to molarity:
-
Molality (m): Molality is defined as the number of moles of solute per kilogram of solvent, unlike molarity, which uses the volume of the solution. Molality is less temperature-dependent than molarity.
-
Normality (N): Normality expresses concentration in terms of gram-equivalent weights per liter of solution. It is particularly useful in acid-base and redox reactions.
-
Mole Fraction (χ): Mole fraction represents the ratio of the number of moles of a particular component to the total number of moles in the solution.
-
Mass Percent (% w/w): Mass percent expresses concentration as the mass of solute (in grams) per 100 grams of solution.
-
Volume Percent (% v/v): Volume percent is used for liquid solutions and expresses the volume of solute (in mL) per 100 mL of solution.
Conclusion: Molarity as a Foundation of Quantitative Chemistry
Molarity is a cornerstone of quantitative chemistry, offering a simple yet powerful way to express solution concentration. Its widespread use stems from its direct connection between the amount of solute and the volume of the solution. Understanding molarity, its calculation, its applications, and its limitations is fundamental for success in chemistry and various related fields. While temperature dependence and volume changes pose some limitations, the advantages of using molarity significantly outweigh these drawbacks, solidifying its place as a critical tool for chemists and scientists worldwide. The related concepts discussed further enhance our ability to express and understand solution concentrations, enabling more precise and accurate analyses in diverse applications.
Latest Posts
Latest Posts
-
Who Proposed The Planetary Model Of The Atom
Mar 21, 2025
-
Which Three Dimensional Figure Is Formed By The Rotation Given
Mar 21, 2025
-
75 Percent Of What Number Is 15
Mar 21, 2025
-
Time Magazine Person Of The Century 1999
Mar 21, 2025
-
Ground State Electron Configuration For Titanium
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Moles Of Solute Per Liter Of Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
