Who Proposed The Planetary Model Of The Atom
News Leon
Mar 21, 2025 · 6 min read
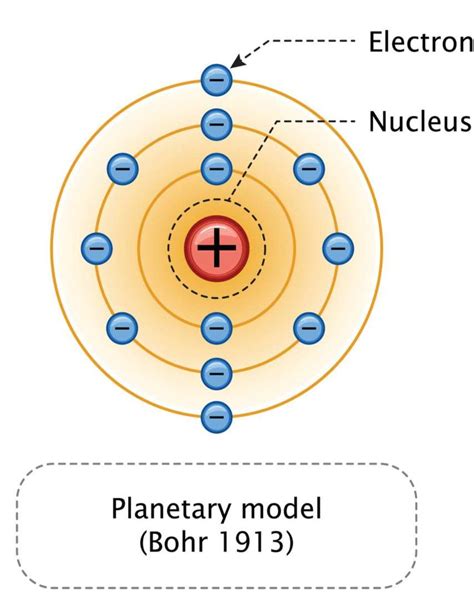
Table of Contents
Who Proposed the Planetary Model of the Atom? A Deep Dive into Atomic Theory
The image of the atom as a miniature solar system, with electrons orbiting a central nucleus, is iconic. This "planetary model" is deeply ingrained in our understanding of atomic structure. But the creation of this model wasn't a single "eureka" moment; it was the culmination of decades of scientific investigation, building upon earlier theories and experimental evidence. Understanding who proposed this model requires exploring the scientific journey that led to its development. This journey involves several key figures, each contributing crucial pieces to the puzzle. While Ernest Rutherford is most often credited with the planetary model, the complete picture requires acknowledging the contributions of J.J. Thomson and the subsequent refinements made by Niels Bohr.
J.J. Thomson and the Plum Pudding Model: A Precursor to the Planetary Model
Before we delve into Rutherford's revolutionary work, it's crucial to understand the prevailing atomic model of his time: J.J. Thomson's "plum pudding" model. In 1897, Thomson discovered the electron, a negatively charged particle significantly smaller than the atom itself. This discovery shattered the then-accepted view of the atom as an indivisible, solid sphere.
Thomson's model, proposed around 1904, posited that the atom was a sphere of positive charge, with negatively charged electrons embedded within it, like plums in a pudding. This model successfully explained some experimental observations, such as the atom's overall neutrality (equal positive and negative charges). However, it lacked a clear structure and couldn't account for the growing body of experimental data emerging from scattering experiments.
Key Takeaways from Thomson's Work:
- Discovery of the electron: This was a groundbreaking discovery that fundamentally changed the understanding of the atom.
- Plum Pudding Model: While inaccurate in its depiction of atomic structure, it served as an important stepping stone towards more accurate models.
- Foundation for future discoveries: Thomson's work laid the groundwork for the experiments that would eventually lead to the planetary model.
Ernest Rutherford's Gold Foil Experiment and the Birth of the Planetary Model
Ernest Rutherford, a former student of Thomson, conducted a series of experiments in 1909 that would revolutionize atomic theory. These experiments involved bombarding a thin gold foil with alpha particles, positively charged particles emitted by radioactive substances. Rutherford's team, including Hans Geiger and Ernest Marsden, expected the alpha particles to pass through the foil with only minor deflections, consistent with Thomson's plum pudding model.
The results were astonishing. While most alpha particles did pass through undeflected, a small but significant number were deflected at large angles, some even bouncing straight back. This unexpected observation couldn't be explained by the plum pudding model. Rutherford famously remarked that it was "as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you."
Based on these results, Rutherford proposed a new model of the atom in 1911. This model posited that:
- The atom is mostly empty space.
- Almost all of the atom's positive charge and mass are concentrated in a tiny, dense region at the center, which he called the nucleus.
- The negatively charged electrons orbit the nucleus at a relatively large distance.
This model, now known as the planetary model, was a radical departure from Thomson's model. It accurately explained the scattering of alpha particles: the large deflections resulted from the alpha particles encountering the dense, positively charged nucleus.
Key Takeaways from Rutherford's Work:
- Discovery of the nucleus: This was a monumental discovery that completely redefined our understanding of the atom's structure.
- Planetary Model: Rutherford's model provided the first accurate representation of the atom's structure, albeit with limitations.
- Experimental validation: The gold foil experiment provided strong experimental evidence supporting the planetary model.
Limitations of Rutherford's Planetary Model and the Entrance of Niels Bohr
Rutherford's planetary model, while a significant advancement, had a major flaw. According to classical electromagnetism, an accelerating charged particle, like an electron orbiting a nucleus, should continuously emit electromagnetic radiation, losing energy and eventually spiraling into the nucleus. This would make atoms unstable, which contradicts experimental observations.
This is where Niels Bohr entered the scene. Building upon Rutherford's work, Bohr proposed a modified model in 1913, incorporating concepts from quantum theory. Bohr's model addressed the instability problem by postulating that:
- Electrons orbit the nucleus in specific, quantized energy levels or shells.
- Electrons can only jump between these energy levels by absorbing or emitting photons of specific energies.
- While orbiting in these specific energy levels, electrons do not radiate energy.
Bohr's model successfully explained the discrete spectral lines observed in the hydrogen atom's emission spectrum. The specific energy levels correspond to specific wavelengths of light emitted when electrons transition between them.
Key Takeaways from Bohr's Work:
- Quantization of energy levels: This was a crucial step in bridging classical physics with quantum mechanics.
- Explanation of atomic spectra: Bohr's model provided a successful explanation for the observed spectral lines of hydrogen.
- Refinement of the planetary model: Bohr's model addressed the major limitations of Rutherford's model, making it more accurate and consistent with experimental data.
The Evolution Beyond the Planetary Model
While Bohr's model was a significant improvement, it still had limitations. It couldn't accurately predict the spectra of atoms with more than one electron and didn't account for the wave-particle duality of electrons. The development of quantum mechanics in the 1920s and 1930s led to even more sophisticated models of the atom, such as the quantum mechanical model, which describes electrons as probability distributions rather than orbiting particles.
Despite its limitations, Rutherford's planetary model remains a cornerstone of atomic theory. It provided the crucial framework for understanding the atom's fundamental structure: a dense, positively charged nucleus surrounded by orbiting electrons. Bohr's refinement incorporated quantum principles, resolving the instability problem and successfully explaining atomic spectra. While subsequent models have provided a more nuanced and accurate description of the atom, the legacy of Rutherford and Bohr's contributions to the planetary model continues to shape our understanding of the atomic world. The visual representation of the atom with orbiting electrons, though a simplification, continues to serve as a powerful and accessible illustration of atomic structure for educational purposes.
The Importance of Acknowledging All Contributions:
It’s important to note that attributing the planetary model solely to Rutherford would be an oversimplification. Thomson’s discovery of the electron and the development of his plum pudding model, albeit ultimately incorrect, provided the groundwork for Rutherford's groundbreaking experiments. Bohr's crucial refinement of the model incorporated quantum mechanics, leading to a more complete and accurate understanding. The evolution of atomic theory showcases the collaborative and iterative nature of scientific progress, a testament to the collective effort of numerous scientists building upon each other's discoveries. The "planetary model" represents a significant milestone in this journey, and understanding its development requires appreciating the contributions of these key figures – Thomson, Rutherford, and Bohr.
Latest Posts
Related Post
Thank you for visiting our website which covers about Who Proposed The Planetary Model Of The Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.