Lewis Dot Diagram For Fluoride Ion
News Leon
Apr 05, 2025 · 6 min read
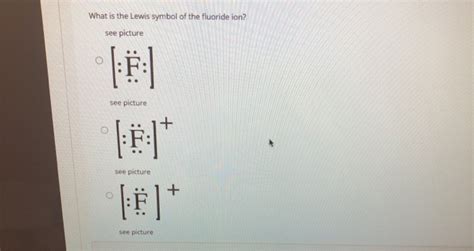
Table of Contents
Lewis Dot Diagram for Fluoride Ion: A Comprehensive Guide
The fluoride ion (F⁻) is a simple but crucial species in chemistry, representing a fundamental concept in bonding and chemical structure. Understanding its Lewis dot diagram is essential for grasping many core chemical principles. This comprehensive guide delves deep into the creation and interpretation of the Lewis dot structure for the fluoride ion, exploring its implications for various chemical phenomena. We'll go beyond the basics, exploring its electronic configuration, octet rule satisfaction, and its role in various chemical interactions.
Understanding Lewis Dot Diagrams
Before diving into the specifics of the fluoride ion, let's refresh our understanding of Lewis dot diagrams. These diagrams, also known as Lewis structures or electron dot structures, are visual representations of the valence electrons of atoms and molecules. Valence electrons are the outermost electrons, those most involved in chemical bonding. The Lewis dot diagram provides a simple and effective way to visualize the arrangement of these valence electrons, helping us predict bonding behavior and molecular geometry.
Key Components of a Lewis Dot Diagram:
- Element Symbol: The symbol of the element is placed in the center of the diagram.
- Valence Electrons: Dots are used to represent the valence electrons surrounding the element symbol. Each dot represents a single electron. Dots are typically placed individually around the symbol before pairing up.
Determining the Valence Electrons of Fluorine
Fluorine (F) is a halogen, belonging to Group 17 (VIIA) of the periodic table. Elements in Group 17 have seven valence electrons. This means a neutral fluorine atom has seven electrons in its outermost shell. These seven electrons are distributed in its 2s and 2p orbitals.
Constructing the Lewis Dot Diagram for Neutral Fluorine
To draw the Lewis dot diagram for a neutral fluorine atom, we place the element symbol 'F' in the center. Then, we add seven dots around the symbol, representing the seven valence electrons. It's conventional to place one dot on each side before pairing them up. This would look like this:
.
. F .
.
.
.
Formation of the Fluoride Ion (F⁻)
Fluorine, being highly electronegative, readily gains an electron to achieve a stable octet (eight valence electrons). This electron gain transforms the neutral fluorine atom into a fluoride ion (F⁻). The extra electron completes the outermost shell, resulting in a filled octet.
Lewis Dot Diagram for the Fluoride Ion (F⁻)
The Lewis dot structure for the fluoride ion reflects this addition of an electron. We add an eighth dot to the neutral fluorine's Lewis structure. It's common practice to pair this electron with one of the existing single dots, fulfilling the octet rule. The Lewis dot diagram for F⁻ is:
.
:F:
.
or more simply represented as:
[:F:]⁻
The square brackets indicate the ion, and the superscript minus sign denotes the negative charge.
The Octet Rule and the Fluoride Ion
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight electrons in their outermost shell. The formation of the fluoride ion perfectly exemplifies the octet rule. By gaining one electron, fluorine achieves a stable octet, making the fluoride ion a much more stable species than the neutral fluorine atom.
Significance of the Fluoride Ion
The fluoride ion plays a crucial role in numerous contexts:
1. Biological Significance:
- Dental Health: Fluoride ions are vital for dental health. They strengthen tooth enamel by replacing hydroxyl ions (OH⁻) in the hydroxyapatite crystal structure, forming fluorapatite, a more resistant mineral to acid attacks. This helps prevent tooth decay and cavities.
- Bone Health: Fluoride also plays a role in bone metabolism, although the exact mechanisms are complex and still under research. It can enhance bone density in certain cases.
2. Chemical Applications:
- Fluorinated Compounds: Fluoride ions are precursors to many important fluorinated compounds, including fluorocarbons, used as refrigerants and propellants (although many fluorocarbons are now being phased out due to environmental concerns).
- Inorganic Chemistry: Fluoride is a common ligand in coordination complexes, often forming strong bonds with metal ions.
- Industrial Applications: It finds use in various industrial processes, such as etching glass and in the production of certain polymers.
3. Environmental Aspects:
- Water Fluoridation: Controlled addition of fluoride ions to drinking water (water fluoridation) has been a successful public health measure in preventing dental caries in many countries. However, the optimal level of fluoridation remains a subject of ongoing debate.
- Environmental Pollution: Excessive fluoride in water sources can lead to fluorosis, a condition causing discoloration and mottling of teeth and potentially skeletal fluorosis.
Ionic Bonding and the Fluoride Ion
The formation of the fluoride ion is a classic example of ionic bonding. Ionic bonding occurs when there is a significant difference in electronegativity between two atoms. Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. Fluorine has the highest electronegativity of all elements. When fluorine reacts with a metal, such as sodium (Na), the high electronegativity of fluorine causes it to attract an electron from the sodium atom. This results in the formation of a positively charged sodium ion (Na⁺) and a negatively charged fluoride ion (F⁻). The electrostatic attraction between these oppositely charged ions forms an ionic bond, leading to the formation of sodium fluoride (NaF).
Comparing Fluorine Atom and Fluoride Ion: A Summary
| Feature | Fluorine Atom (F) | Fluoride Ion (F⁻) |
|---|---|---|
| Number of Electrons | 9 | 10 |
| Number of Protons | 9 | 9 |
| Charge | 0 | -1 |
| Electronic Configuration | 1s²2s²2p⁵ | 1s²2s²2p⁶ |
| Lewis Dot Diagram | . <br> .F. <br> . <br> . <br> . | [:F:]⁻ |
| Octet Rule | Not satisfied | Satisfied |
| Stability | Low | High |
Beyond the Basics: Advanced Concepts
The Lewis dot diagram provides a foundational understanding of the fluoride ion. However, a deeper understanding requires exploring more advanced concepts:
- Formal Charge: While the Lewis dot structure accurately shows the total number of valence electrons, it doesn’t always represent the most stable arrangement. Formal charge calculations can help determine the most likely structure by considering the distribution of electrons.
- Molecular Orbital Theory: This theory provides a more sophisticated description of bonding than the simple Lewis dot model, considering the interactions of atomic orbitals to form molecular orbitals.
- Crystal Structure: In solid compounds, like sodium fluoride, the fluoride ions occupy specific positions within the crystal lattice, contributing to the overall structure and properties of the solid.
Conclusion
The Lewis dot diagram for the fluoride ion is a simple yet powerful tool for understanding the basic structure and reactivity of this important species. This diagram highlights the key role of the octet rule in chemical bonding and explains the stability of the fluoride ion. By understanding the fluoride ion's Lewis structure, we can appreciate its widespread significance in biological systems, chemical applications, and environmental contexts. This detailed explanation goes beyond a simple representation, providing a comprehensive overview that solidifies your understanding of fundamental chemical principles. The additional discussion of ionic bonding, advanced concepts, and applications broadens your perspective, making this guide invaluable for students and professionals alike seeking a deeper understanding of this fundamental chemical ion.
Latest Posts
Latest Posts
-
What Are Some Examples Of Pulleys
Apr 05, 2025
-
Which Of The Following Pairings Is Correct
Apr 05, 2025
-
Where Is Most Of The Atp Made During Cellular Respiration
Apr 05, 2025
-
Multicellular Eukaryotes That Have Cell Walls And Are Heterotrophic
Apr 05, 2025
-
28 Rounded To The Nearest Ten
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about Lewis Dot Diagram For Fluoride Ion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
