Isotopes Of An Element Have The Same Number Of
News Leon
Mar 20, 2025 · 6 min read
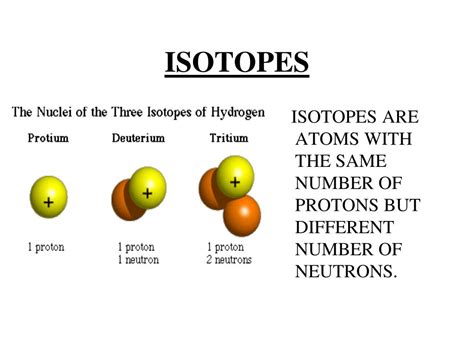
Table of Contents
Isotopes of an Element Have the Same Number of Protons: A Deep Dive into Isotopic Variations
Isotopes are variations of a chemical element that possess the same number of protons but differ in the number of neutrons within their atomic nuclei. This fundamental characteristic defines isotopes and underpins their unique properties and applications across various scientific fields. Understanding the concept of isotopes is crucial for comprehending nuclear chemistry, radioactive decay, and the diverse applications of isotopes in medicine, industry, and research. This article delves into the intricacies of isotopes, exploring their defining characteristics, variations, applications, and significance in our understanding of the natural world.
Understanding the Atomic Structure: Protons, Neutrons, and Electrons
Before delving into the specifics of isotopes, it's essential to revisit the fundamental building blocks of an atom: protons, neutrons, and electrons. The atomic nucleus, situated at the atom's center, houses protons and neutrons. Protons carry a positive charge, while neutrons are electrically neutral. Electrons, negatively charged particles, orbit the nucleus in shells or energy levels.
The atomic number of an element is determined by the number of protons in its nucleus. This number uniquely identifies each element on the periodic table. For instance, all atoms with one proton are hydrogen, while those with six protons are carbon. This number remains constant for a given element, regardless of its isotopic variations.
The mass number of an atom is the total number of protons and neutrons in its nucleus. This number can vary between isotopes of the same element because the number of neutrons can differ.
Defining Isotopes: Same Protons, Different Neutrons
Isotopes of an element are atoms with the same atomic number (number of protons) but differing mass numbers (total number of protons and neutrons). The differing number of neutrons results in variations in the atom's mass and, in some cases, its stability. These variations in neutron number lead to different isotopic forms of the same element.
For example, carbon has three naturally occurring isotopes:
- Carbon-12 (¹²C): 6 protons and 6 neutrons
- Carbon-13 (¹³C): 6 protons and 7 neutrons
- Carbon-14 (¹⁴C): 6 protons and 8 neutrons
Notice that all three isotopes have six protons, defining them as carbon. However, the number of neutrons differs, resulting in variations in their mass numbers and properties. ¹⁴C, for instance, is radioactive, while ¹²C and ¹³C are stable.
Isotopic Notation and Representation
Isotopes are typically represented using isotopic notation, which clearly indicates the element's symbol, mass number, and atomic number. The general format is:
<sup>A</sup><sub>Z</sub>X
Where:
- X is the element's symbol (e.g., C for carbon, O for oxygen).
- Z is the atomic number (number of protons).
- A is the mass number (number of protons + neutrons).
Using carbon-12 as an example, the isotopic notation would be: <sup>12</sup><sub>6</sub>C.
Isotopic Abundance and Average Atomic Mass
Isotopes of an element often exist in different proportions in nature. This is known as isotopic abundance. The relative abundance of each isotope influences the element's average atomic mass, which is a weighted average of the masses of all its isotopes, considering their natural abundances. The average atomic mass is the value reported on the periodic table.
For instance, carbon's average atomic mass is approximately 12.011 amu (atomic mass units), reflecting the higher abundance of ¹²C compared to ¹³C and the trace amount of ¹⁴C.
Isotope Stability and Radioactivity
Not all isotopes are stable. Some isotopes are radioactive, meaning their nuclei are unstable and undergo radioactive decay, emitting particles or energy to transform into a more stable configuration. This decay process can involve alpha emission, beta emission, gamma emission, or other types of radioactive decay.
Radioactive isotopes, also known as radioisotopes, have significant applications in various fields, including:
- Medical imaging and treatment: Radioisotopes like technetium-99m are used in diagnostic imaging (e.g., SPECT scans), while others, such as iodine-131, are used in cancer therapy.
- Industrial applications: Radioisotopes are employed in gauging thickness, tracing materials, and sterilizing medical equipment.
- Archaeological dating: Carbon-14 dating utilizes the decay of ¹⁴C to determine the age of organic materials.
- Geological dating: Radioactive isotopes like uranium-238 and potassium-40 are used to date rocks and minerals, providing insights into Earth's history.
Isotope Separation Techniques
Separating isotopes from one another is a challenging task because they have almost identical chemical properties. However, their slight mass differences allow for separation using techniques like:
- Gaseous diffusion: This method exploits the differences in diffusion rates of gases containing different isotopes. Lighter isotopes diffuse faster than heavier ones.
- Centrifugation: This technique utilizes centrifugal force to separate isotopes based on their mass differences. Heavier isotopes tend to migrate towards the outer edge of the centrifuge.
- Laser isotope separation: This advanced method utilizes lasers to selectively excite and ionize specific isotopes, allowing for their separation.
Applications of Isotopes Across Diverse Fields
Isotopes, both stable and radioactive, have found widespread applications across numerous scientific and technological disciplines. Some notable examples include:
Medicine
- Nuclear medicine: Radioactive isotopes are essential in diagnostic imaging techniques like PET (Positron Emission Tomography) and SPECT (Single-Photon Emission Computed Tomography) scans, allowing doctors to visualize internal organs and detect abnormalities. They are also used in radiotherapy to target and destroy cancer cells.
- Metabolic tracing: Stable isotopes are used as tracers to study metabolic processes in the body. By labeling molecules with stable isotopes, researchers can track their pathways and understand metabolic reactions.
Industry
- Industrial gauging: Radioactive isotopes are used to measure the thickness of materials like paper and metal sheets in manufacturing processes.
- Material tracing: Isotopes are used to trace the flow of materials in industrial processes, helping to optimize efficiency and reduce waste.
- Sterilization: Gamma radiation from radioisotopes is used to sterilize medical equipment and food products.
Environmental Science
- Environmental tracing: Isotopes are used to trace the movement of water, pollutants, and other substances in the environment.
- Climate change research: Isotopic analysis of ice cores and sediments helps scientists reconstruct past climates and understand the impact of human activities on the environment.
Archaeology and Geology
- Radiocarbon dating: Carbon-14 dating is a crucial technique for determining the age of organic materials, providing valuable insights into past civilizations and events.
- Geological dating: Radioactive isotopes are used to determine the age of rocks and minerals, enabling scientists to reconstruct geological timelines and understand Earth's history.
Conclusion: The Significance of Isotopes in Science and Technology
Isotopes, with their variations in neutron number while maintaining the same number of protons, play a vital role in numerous scientific and technological fields. Their unique properties, whether stable or radioactive, allow for a wide range of applications, from medical diagnostics and treatment to industrial processes and scientific research. Understanding the concept of isotopes is fundamental to grasping nuclear chemistry, radioactive decay, and the significance of these variations in shaping our understanding of the natural world and driving technological advancements. The continued development and refinement of isotopic techniques promise further advancements in numerous areas, offering new opportunities for innovation and discovery. From unraveling the mysteries of the universe to improving healthcare and advancing industrial processes, isotopes remain a cornerstone of scientific progress and technological innovation.
Latest Posts
Latest Posts
-
Which Of The Following Is Not A Protein Function
Mar 20, 2025
-
Explain How Ionic Compounds Dissolve In Water
Mar 20, 2025
-
The Additional Satisfaction Of Consuming A Good Or Service Is
Mar 20, 2025
-
Which Of The Following Organisms Has No Specialized Respiratory Structures
Mar 20, 2025
-
What Is The Latin Word For Sour
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Isotopes Of An Element Have The Same Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
