Is Water Good Conductor Of Heat
News Leon
Mar 22, 2025 · 5 min read
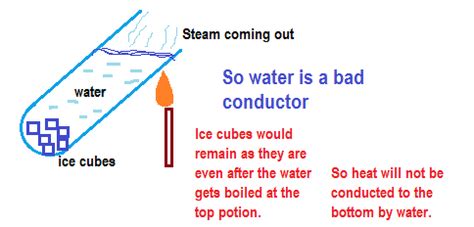
Table of Contents
Is Water a Good Conductor of Heat? Exploring the Thermal Properties of H₂O
Water, the elixir of life, is a substance we interact with constantly. We drink it, bathe in it, and utilize it in countless industrial and domestic applications. But beyond its vital biological role, water possesses fascinating physical properties, one of which is its thermal conductivity. The question, "Is water a good conductor of heat?" isn't a simple yes or no. The answer is nuanced and depends on several factors, including temperature, pressure, and the presence of impurities. This comprehensive exploration will delve into the thermal properties of water, examining why it's a relatively poor conductor compared to many other materials, while still playing a crucial role in heat transfer processes.
Understanding Heat Transfer Mechanisms
Before we dissect water's heat conductivity, let's briefly review the fundamental mechanisms of heat transfer:
-
Conduction: This involves the direct transfer of heat energy through a material, from molecule to molecule. This is the primary mechanism we'll be focusing on when considering water's heat conductivity. In solids, tightly packed molecules readily transfer energy.
-
Convection: Heat transfer through the movement of fluids (liquids or gases). Warmer, less dense fluid rises, while cooler, denser fluid sinks, creating a cycle of heat transfer. Water, being a fluid, demonstrates significant convective heat transfer.
-
Radiation: Heat transfer through electromagnetic waves. Water absorbs and emits infrared radiation, but this mechanism is less dominant than conduction and convection in most scenarios involving water's heat transfer.
Water's Molecular Structure and Heat Conduction
Water's unique molecular structure significantly influences its thermal conductivity. Each water molecule (H₂O) is polar, meaning it has a slightly positive and slightly negative end due to the uneven distribution of charge. These polar molecules are attracted to each other through hydrogen bonds, creating a relatively strong intermolecular force.
While hydrogen bonds contribute to water's high boiling point and surface tension, they also hinder its ability to conduct heat efficiently. The strong hydrogen bonds restrict the free movement of molecules, limiting the speed at which kinetic energy (heat) can be transferred from one molecule to the next through conduction. This is in contrast to materials like metals, where electrons are free to move and readily transfer heat energy.
Comparing Water's Conductivity to Other Substances
To understand how good (or bad) water is as a heat conductor, we can compare its thermal conductivity to other common materials:
| Material | Thermal Conductivity (W/m·K) |
|---|---|
| Copper | 401 |
| Aluminum | 237 |
| Steel | 50 |
| Glass | 0.8 |
| Water (20°C) | 0.6 |
| Air | 0.025 |
As the table illustrates, water's thermal conductivity is significantly lower than that of metals like copper and aluminum, which are excellent conductors. It's even slightly lower than glass. However, it's considerably better than air, a very poor conductor. This highlights water's relatively poor conductive properties compared to many solids but superior ability compared to gases.
Factors Affecting Water's Heat Conductivity
Several factors can influence the thermal conductivity of water:
Temperature:
Water's thermal conductivity increases slightly with temperature. This is because higher temperatures increase molecular kinetic energy, leading to slightly more efficient energy transfer. However, the change is relatively small compared to the impact of other factors.
Pressure:
Increased pressure generally leads to a minor increase in water's thermal conductivity. Higher pressure reduces the intermolecular distance, potentially facilitating slightly better energy transfer.
Impurities:
The presence of dissolved substances (salts, minerals, etc.) can alter water's thermal conductivity. Some impurities can slightly increase conductivity while others may have a negligible effect or even slightly decrease it, depending on their nature and concentration. This effect is typically much less significant than the inherent limitations imposed by the hydrogen bonding.
State of Water:
The state of water (solid, liquid, gas) dramatically impacts its thermal conductivity. Ice (solid water) has lower thermal conductivity than liquid water. Water vapor (gaseous water) possesses the lowest thermal conductivity of the three states.
The Role of Convection in Water's Heat Transfer
While water is a relatively poor conductor of heat, its convective properties are highly significant. When water is heated, the warmer, less dense water rises, creating convection currents. These currents efficiently transfer heat throughout the water body. This is why, for example, heating a pot of water on a stove involves both conduction (heat transferring from the pot to the water's bottom layer) and convection (heat distributing throughout the water through rising hot water and sinking cool water). Convection plays a far more important role than conduction in distributing heat within a large body of water.
Applications and Implications
The thermal properties of water have important implications across diverse fields:
-
Climate Regulation: Water's high heat capacity and its role in convection play crucial roles in regulating Earth's climate. Oceans act as massive heat reservoirs, moderating temperature fluctuations.
-
Cooling Systems: Water's relatively good heat capacity (the amount of heat required to raise its temperature) makes it an effective coolant in industrial and automotive applications.
-
Cooking: Understanding water's thermal properties is essential for efficient and safe cooking. The combination of conduction and convection allows for even heating of food in water-based cooking methods.
-
Heating Systems: Water is widely used in central heating systems, its ability to absorb and distribute heat making it an effective heat transfer medium.
-
Biological Systems: Water's thermal properties are critical for maintaining stable temperatures within living organisms. Its high heat capacity helps to buffer against temperature fluctuations, protecting cells from damage.
Conclusion: Water's Complex Thermal Behavior
In conclusion, the question of whether water is a good conductor of heat is multifaceted. Compared to metals, water is a poor conductor of heat. Its relatively low thermal conductivity is primarily due to the strong hydrogen bonds between its polar molecules, which hinder the efficient transfer of kinetic energy through direct molecular interactions. However, water's convective heat transfer capabilities are exceptionally high, making it an extremely efficient medium for heat distribution in many applications. The interplay between conduction and convection, influenced by factors like temperature, pressure, and impurities, determines its overall effectiveness in heat transfer processes. Understanding these properties is vital across a range of scientific disciplines and engineering applications. Water’s thermal behavior is a testament to its unique molecular structure and its indispensable role in our world.
Latest Posts
Latest Posts
-
52 Rounded To The Nearest Ten
Mar 22, 2025
-
What Is The Product Of A Number And Its Reciprocal
Mar 22, 2025
-
20 Meters On A Basketball Court
Mar 22, 2025
-
One Billion Equals How Many Crores
Mar 22, 2025
-
A Goverment With A Small Group That Holds Poer
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Is Water Good Conductor Of Heat . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
