Is Water A Good Conductor Of Heat
News Leon
Mar 16, 2025 · 5 min read
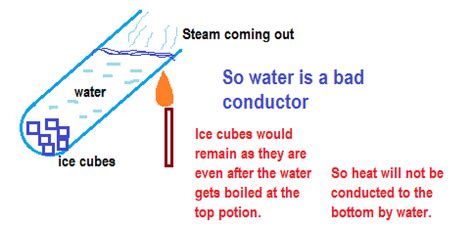
Table of Contents
Is Water a Good Conductor of Heat? Exploring the Thermal Properties of H₂O
Water, the elixir of life, plays a crucial role in countless natural processes and technological applications. Understanding its thermal properties, particularly its heat conductivity, is essential for comprehending these diverse roles. While often perceived as a relatively poor conductor of heat compared to metals, water's thermal behavior is more nuanced and fascinating than a simple "yes" or "no" answer can convey. This comprehensive exploration delves into the intricacies of water's heat conductivity, examining its mechanisms, influencing factors, and implications across various fields.
Understanding Heat Transfer Mechanisms in Water
Before directly addressing water's conductivity, let's clarify the fundamental mechanisms of heat transfer: conduction, convection, and radiation. These processes dictate how thermal energy moves from a hotter region to a cooler one.
Conduction: Molecular Vibrations
Heat conduction involves the direct transfer of thermal energy through molecular vibrations and collisions. In solids, tightly packed atoms and molecules readily transmit vibrations, resulting in efficient heat conduction. Liquids, while less structured, also exhibit conduction, albeit less efficiently than solids. In water, the relatively strong hydrogen bonds between water molecules influence the rate of energy transfer through these vibrations. However, compared to metals with their freely moving electrons, the mechanism is slower.
Convection: Bulk Movement of Fluids
Convection is the dominant heat transfer mechanism in fluids (liquids and gases). It involves the bulk movement of heated fluid, which rises due to its decreased density, carrying thermal energy with it. Cooler fluid then replaces the warmer fluid, creating a cycle of movement. Convection plays a significant role in water's apparent heat transfer, often overshadowing the contribution of pure conduction.
Radiation: Electromagnetic Waves
Radiation involves the emission and absorption of electromagnetic waves, which can travel through a vacuum. Water molecules absorb and emit infrared radiation, contributing to heat transfer, but this mechanism is less significant than conduction and convection in most scenarios involving water.
Water's Heat Capacity: A Key Player
Water possesses an exceptionally high specific heat capacity. This means it requires a significant amount of heat energy to raise its temperature by a given amount. This property is crucial in understanding its apparent "poor" conductivity. While it doesn't conduct heat quickly through conduction alone, it can absorb and store a large amount of heat energy, making it an excellent heat buffer or thermal insulator in certain contexts.
Factors Influencing Water's Heat Conductivity
Several factors significantly impact the rate at which heat transfers through water:
Temperature:
Water's thermal conductivity increases slightly with temperature. This is because higher temperatures lead to increased molecular kinetic energy and more frequent collisions, facilitating the transfer of thermal energy through conduction.
Pressure:
Increased pressure slightly enhances water's thermal conductivity. This is because higher pressure leads to a denser packing of water molecules, promoting more efficient energy transfer through collisions.
Salinity:
Adding dissolved salts to water alters its conductivity. Generally, increasing salinity slightly increases thermal conductivity due to the interactions between ions and water molecules. However, the impact is less significant than temperature or pressure changes.
Presence of Other Substances:
The presence of other substances suspended in water significantly impacts its thermal conductivity. For instance, the addition of fine particles can affect the convection currents, and the presence of dissolved gases can alter the overall thermal properties.
Comparing Water's Conductivity to Other Substances
To put water's heat conductivity into perspective, let's compare it to other common substances:
| Substance | Thermal Conductivity (W/m·K) |
|---|---|
| Copper | 401 |
| Aluminum | 237 |
| Steel | 50 |
| Water (20°C) | 0.6 |
| Air | 0.026 |
As the table shows, water's thermal conductivity is significantly lower than that of metals like copper and aluminum, highlighting its relatively poor conductive ability. However, it's considerably higher than that of air, explaining its effectiveness as a coolant in various applications.
The Role of Convection in Water's Apparent Heat Transfer
As previously mentioned, convection plays a dominant role in how we perceive water's heat transfer. When heating a pot of water, the heated water at the bottom rises, creating convection currents that distribute heat throughout the liquid. This convective heat transfer is far more efficient than the conductive transfer in water itself. Therefore, while water is not a good conductor in the sense of direct molecular energy transfer, it facilitates efficient overall heat transfer through convection.
Applications Leveraging Water's Thermal Properties
Water's unique thermal properties are exploited in various applications:
Cooling Systems:
Water's high specific heat capacity and relatively good heat transfer (aided by convection) makes it an ideal coolant in numerous industrial and technological applications, including car engines, power plants, and computer cooling systems.
Climate Regulation:
Ocean currents and the vast heat capacity of oceans play a crucial role in regulating global climate patterns. The oceans absorb and release immense amounts of heat energy, moderating temperature fluctuations across the planet.
Heating and Cooling Buildings:
Water-based heating and cooling systems (hydronic systems) are widely used in buildings due to water's efficient heat transfer capabilities.
Food Preparation:
Water's high heat capacity and ability to facilitate heat transfer through convection are crucial in many cooking processes, from boiling to steaming.
Industrial Processes:
Water is used extensively in various industrial processes that require efficient heat transfer and temperature control.
Conclusion: Nuance and Context are Key
The question "Is water a good conductor of heat?" doesn't have a simple yes or no answer. While its pure conductive ability is relatively low compared to metals, its high specific heat capacity and the dominant role of convection make it an efficient medium for overall heat transfer in many situations. Understanding the interplay between conduction, convection, and water's other thermal properties is critical for appreciating its role in various natural and engineered systems. The context – the specific application and the processes involved – determines whether water's thermal properties are advantageous or not. Its ability to absorb, store, and transfer heat effectively positions it as a crucial substance in countless vital processes. Its seemingly simple chemical makeup belies a complex and fascinating thermal behavior that continues to be a subject of ongoing scientific investigation.
Latest Posts
Latest Posts
-
An Earth Satellite Moves In A Circular Orbit
Mar 17, 2025
-
What Is The Value Of K In Physics
Mar 17, 2025
-
The Study Of Tissues With A Microscope Is Called
Mar 17, 2025
-
The Male Gamete Is Called The
Mar 17, 2025
-
The Broad Portion Of The Leaf That Carries Out Photosynthesis
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is Water A Good Conductor Of Heat . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
