Is Temperature An Extensive Or Intensive Property
News Leon
Mar 28, 2025 · 5 min read
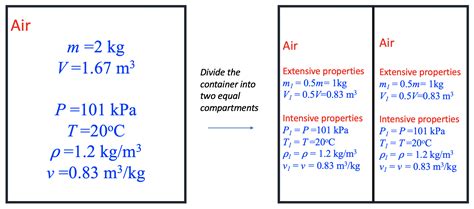
Table of Contents
Is Temperature an Extensive or Intensive Property? A Deep Dive
Understanding the difference between extensive and intensive properties is crucial in various fields, from thermodynamics and chemistry to materials science and engineering. This article delves deep into the nature of temperature, exploring whether it's an extensive or intensive property, and clarifying the subtle distinctions that often cause confusion. We will unpack the definitions, provide examples, and ultimately answer the central question definitively.
Extensive vs. Intensive Properties: A Fundamental Distinction
Before we tackle temperature, let's establish a clear understanding of the key difference between extensive and intensive properties. This forms the bedrock of our investigation.
Extensive properties are those that depend on the amount or size of matter present. In simpler terms, if you double the amount of a substance, the extensive property also doubles. Classic examples include:
- Mass: The mass of 2 kilograms of water is double the mass of 1 kilogram of water.
- Volume: Similarly, the volume of 2 liters of water is twice that of 1 liter.
- Length: A 2-meter-long rod is twice as long as a 1-meter-long rod.
- Energy: The total energy content of a system is directly proportional to its size.
Intensive properties, on the other hand, are independent of the amount of matter. No matter how much of a substance you have, the intensive property remains constant. Examples include:
- Temperature: The temperature of a cup of boiling water is the same as the temperature of a pot of boiling water (both at 100°C at sea level).
- Density: The density of gold remains the same whether you have a gold nugget or a gold bar.
- Pressure: The pressure within a sealed container remains constant regardless of its size (assuming constant temperature).
- Boiling point: The boiling point of water remains 100°C (at sea level) regardless of the amount of water.
- Color: The color of a substance doesn't change with the amount of that substance.
Temperature: A Closer Look
Temperature is a measure of the average kinetic energy of the particles within a system. Whether it's the molecules in a gas, atoms in a solid, or ions in a liquid, the faster these particles move, the higher the temperature. This fundamental understanding is key to deciphering whether temperature is extensive or intensive.
Let's consider two scenarios:
Scenario 1: Combining Two Identical Systems
Imagine you have two identical containers, each filled with 1 liter of water at 25°C. If you combine the contents of these containers into a larger vessel, what happens to the temperature? The temperature remains at 25°C (neglecting minor heat losses to the environment). The total amount of water has doubled, but the temperature, a measure of the average kinetic energy, hasn't changed.
Scenario 2: Different Temperatures
Now, consider two containers, one with 1 liter of water at 25°C and another with 1 liter of water at 50°C. Combining these will result in a final temperature somewhere between 25°C and 50°C – a temperature that's the average of the two initial temperatures (again, neglecting heat loss). This illustrates that the final temperature isn't simply the sum of the initial temperatures; the resulting temperature reflects an average, not a summation.
These scenarios strongly suggest that temperature is intensive, not extensive. Adding more of a substance doesn't change its temperature unless there's an energy exchange with the surroundings (like in the case of mixing water at different temperatures).
The Misconception and its Clarification
The potential confusion arises from the concept of total heat energy. While temperature is intensive, the total heat energy (often calculated as Q = mcΔT) is extensive. The heat energy content does depend on the mass (m) and the temperature change (ΔT) of the substance.
A larger mass of water at a given temperature will possess more heat energy than a smaller mass at the same temperature. However, the temperature itself remains unchanged. The key is differentiating between the average kinetic energy (temperature) and the total kinetic energy (heat energy).
Practical Applications and Implications
Understanding the intensive nature of temperature is crucial in various applications:
- Thermodynamics: Thermodynamic calculations often rely on intensive properties like temperature and pressure to define the state of a system. The intensive nature of temperature ensures that these calculations are independent of the system's size.
- Materials Science: The phase transitions of materials are often dictated by temperature. Knowing that temperature is an intensive property allows engineers and scientists to predict material behavior regardless of the sample size.
- Chemical Engineering: Chemical reactions often depend on temperature. Understanding that temperature is intensive simplifies process scaling in chemical reactors.
- Meteorology: Temperature measurements are used to understand weather patterns. These measurements are meaningful regardless of the volume of air being sampled.
Addressing Common Doubts and Misconceptions
Several points often lead to confusion regarding temperature's intensive nature. Let's address them:
- Heat Transfer: While temperature is intensive, heat transfer is not. The amount of heat transferred between two objects depends on the temperature difference and the mass of the objects involved. This does not contradict the intensive nature of temperature.
- Temperature Gradients: Systems can have temperature gradients, meaning the temperature varies across the system. This doesn't change the fact that temperature at any specific point is an intensive property. The gradient itself can be considered an extensive property, but the temperature at individual points is not.
- Statistical Mechanics: A statistical mechanics approach to temperature reinforces its intensive nature, demonstrating that it's the average energy per particle, independent of the number of particles.
Conclusion: Temperature is Intensive
In summary, temperature is definitively an intensive property. It's a measure of the average kinetic energy of particles, independent of the amount of matter present. While the total heat energy of a system is extensive, the temperature itself remains unchanged by the system's size, making it a crucial intensive property in numerous scientific and engineering disciplines. The distinctions between extensive and intensive properties, and their implications, are fundamental to understanding the behavior of matter and energy. Grasping this crucial difference enhances our ability to analyze and predict the behavior of various systems. Furthermore, understanding this fundamental principle helps avoid common pitfalls in calculations and analysis across different fields. The intensive nature of temperature provides a consistent and reliable parameter in numerous scientific models and applications, making its understanding critical for advancements in diverse fields of study and engineering.
Latest Posts
Latest Posts
-
A Solution Of H2so4 With A Molal Concentration Of
Mar 31, 2025
-
Chitin Is Composed Of Glucose And
Mar 31, 2025
-
Calculate Zeff For A Valence Electron In An Oxygen Atom
Mar 31, 2025
-
Reaction Between Magnesium And Hydrochloric Acid
Mar 31, 2025
-
Is Electric Charge A Vector Quantity
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Is Temperature An Extensive Or Intensive Property . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
