Reaction Between Magnesium And Hydrochloric Acid
News Leon
Mar 31, 2025 · 6 min read
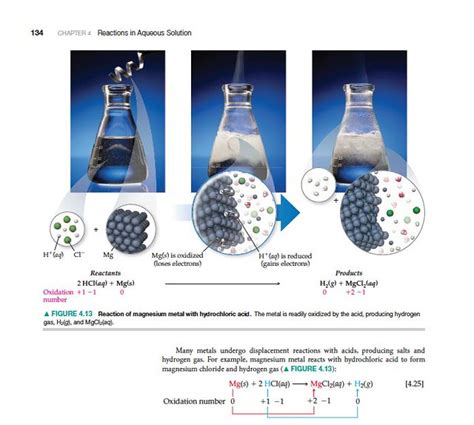
Table of Contents
The Reaction Between Magnesium and Hydrochloric Acid: A Comprehensive Exploration
The reaction between magnesium (Mg) and hydrochloric acid (HCl) is a classic example of a single displacement reaction, frequently used in chemistry demonstrations and experiments to illustrate fundamental chemical principles. This reaction, producing magnesium chloride (MgCl₂) and hydrogen gas (H₂), offers a rich opportunity to explore various aspects of chemistry, from stoichiometry and kinetics to thermodynamics and applications. This comprehensive article delves into the intricacies of this reaction, examining its mechanism, factors influencing its rate, safety precautions, and practical applications.
Understanding the Reaction
The reaction between magnesium and hydrochloric acid is represented by the following balanced chemical equation:
Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g)
This equation shows that one mole of solid magnesium reacts with two moles of aqueous hydrochloric acid to produce one mole of aqueous magnesium chloride and one mole of hydrogen gas. The "(s)", "(aq)", and "(g)" indicate the physical states of the reactants and products: solid, aqueous (dissolved in water), and gas, respectively.
The Mechanism of the Reaction
The reaction proceeds through a series of steps involving the transfer of electrons. The magnesium atom, having a relatively low electronegativity, readily loses two electrons to become a magnesium ion (Mg²⁺). These electrons are accepted by the hydrogen ions (H⁺) present in the hydrochloric acid solution. Each hydrogen ion gains one electron to form a hydrogen atom (H), and two hydrogen atoms combine to form a molecule of hydrogen gas (H₂). This electron transfer process is the core of the reaction and makes it an example of a redox (reduction-oxidation) reaction. Magnesium is oxidized (loses electrons), and hydrogen ions are reduced (gain electrons).
Factors Affecting the Reaction Rate
Several factors can influence the rate at which magnesium reacts with hydrochloric acid. Understanding these factors is crucial for conducting controlled experiments and predicting the outcome of the reaction:
-
Concentration of Hydrochloric Acid: A higher concentration of HCl means a greater number of H⁺ ions available to react with magnesium. This leads to a faster reaction rate because more collisions between magnesium and hydrogen ions occur per unit time.
-
Surface Area of Magnesium: The reaction occurs at the surface of the magnesium metal. Increasing the surface area, such as by using magnesium powder or ribbon instead of a large piece, significantly increases the reaction rate. This is because more magnesium atoms are exposed to the acid, allowing for more simultaneous reactions.
-
Temperature: Increasing the temperature increases the kinetic energy of the reacting particles. This leads to more frequent and energetic collisions between magnesium and hydrogen ions, resulting in a faster reaction rate. The rate generally doubles for every 10°C increase in temperature (approximately, depending on the specific conditions).
-
Presence of Inhibitors: Certain substances can act as inhibitors, slowing down the reaction rate. These inhibitors often work by forming a layer on the surface of the magnesium, preventing it from reacting with the acid.
Observing the Reaction: Experimental Setup and Observations
A simple experiment can demonstrate the reaction between magnesium and hydrochloric acid. The following is a typical setup:
-
Materials: Magnesium ribbon or powder, dilute hydrochloric acid (e.g., 1M or 2M), test tube, test tube rack, gas collection apparatus (optional, for collecting hydrogen gas), thermometer (optional), safety goggles.
-
Procedure: Add a small amount of dilute hydrochloric acid to a test tube. Carefully add a piece of magnesium ribbon or a small amount of magnesium powder to the acid. Observe the reaction closely.
-
Observations: The reaction is readily apparent through several observations:
- Fizzing or effervescence: The production of hydrogen gas is evident through the visible bubbling or fizzing at the magnesium's surface.
- Heat generation: The reaction is exothermic, meaning it releases heat. You'll notice a slight temperature increase in the solution.
- Magnesium dissolution: The magnesium metal gradually dissolves as it reacts with the acid.
- Color change (potential): Depending on the concentration of the acid and the amount of magnesium used, you might observe a slight color change in the solution, typically becoming slightly less clear due to the formation of magnesium chloride.
Safety Precautions
It is crucial to emphasize safety when performing this experiment:
- Always wear safety goggles: This protects your eyes from splashes of acid and hydrogen gas.
- Use dilute acid: Concentrated hydrochloric acid is highly corrosive and dangerous. Always use a dilute solution for this experiment.
- Perform the experiment in a well-ventilated area: Hydrogen gas is flammable, and a build-up of hydrogen gas in an enclosed space can be dangerous.
- Avoid inhaling the hydrogen gas: While not toxic, the gas can be irritating to the respiratory system.
- Proper disposal: After the reaction is complete, dispose of the reaction mixture according to your school or lab's safety guidelines.
Applications of the Reaction
The reaction between magnesium and hydrochloric acid has several practical applications:
-
Hydrogen Gas Production: This reaction is a convenient method for producing small quantities of relatively pure hydrogen gas for laboratory use. While not industrially favored for large-scale production, it's suitable for educational and small-scale experimental settings.
-
Determination of Magnesium Content: The reaction can be used in quantitative analysis to determine the amount of magnesium present in a sample. By measuring the volume of hydrogen gas produced, one can calculate the mass of magnesium that reacted using stoichiometry.
-
Educational Demonstrations: This reaction serves as a valuable tool in teaching fundamental chemical principles such as single displacement reactions, redox reactions, stoichiometry, and reaction kinetics.
-
Chemical Synthesis: Magnesium chloride, a product of this reaction, is used in various industrial applications, including the production of other chemicals, as a de-icing agent, and in the manufacture of some fire retardants.
Further Exploration: Advanced Concepts
The reaction between magnesium and hydrochloric acid provides an excellent springboard for exploring more advanced chemical concepts:
-
Reaction Kinetics: Studying the factors that affect the rate of reaction, such as concentration, temperature, and surface area, allows for a deeper understanding of reaction kinetics and rate laws.
-
Thermodynamics: Calculating the enthalpy change (ΔH) of the reaction using calorimetry provides insight into the thermodynamic aspects of the process, including whether the reaction is exothermic or endothermic and its spontaneity.
-
Electrochemistry: The reaction can be investigated using electrochemical cells, where the reaction occurs in a controlled environment allowing the study of electron transfer processes and the generation of electrical energy.
Conclusion
The reaction between magnesium and hydrochloric acid, seemingly simple on the surface, offers a wealth of learning opportunities. From understanding basic chemical principles to delving into advanced concepts in kinetics and thermodynamics, this reaction serves as a cornerstone in the study of chemistry. However, always remember to prioritize safety when conducting experiments involving chemicals. Through careful observation and analysis, this seemingly simple reaction can reveal a universe of chemical insights. Understanding this fundamental reaction unlocks a deeper appreciation for the principles governing chemical reactions and their diverse applications. Its simplicity makes it ideal for introductory chemistry studies, while its complexity offers challenges and rewards for those seeking a more in-depth understanding of the chemical world.
Latest Posts
Related Post
Thank you for visiting our website which covers about Reaction Between Magnesium And Hydrochloric Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
