Is Steel A Conductor Or Insulator
News Leon
Apr 01, 2025 · 5 min read
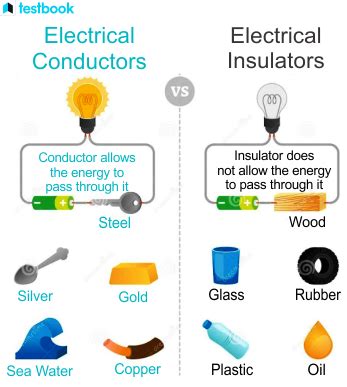
Table of Contents
Is Steel a Conductor or an Insulator? Understanding Electrical Conductivity in Steel
Steel, a ubiquitous material in construction, manufacturing, and countless other applications, often sparks the question: is it a conductor or an insulator? The simple answer is steel is a conductor of electricity, but the complexity lies in understanding the nuances of its conductivity and the factors influencing it. This article delves deep into the electrical properties of steel, exploring its conductive nature, the factors affecting its conductivity, and its practical implications.
The Atomic Structure and Electrical Conductivity
To grasp why steel conducts electricity, we must examine its atomic structure. Steel is an alloy predominantly composed of iron (Fe), with carbon (C) and other elements like manganese (Mn), silicon (Si), and phosphorus (P) present in varying proportions. The key to conductivity lies in the free electrons within the atomic structure of iron.
Iron atoms possess electrons in their outer shells that are loosely bound to the nucleus. These valence electrons are not firmly attached to a particular atom and can move freely throughout the metallic lattice. When an electric field is applied, these delocalized electrons are easily mobilized, creating a flow of electric current. This is the fundamental reason why iron, and consequently steel, are excellent electrical conductors.
The Role of Alloying Elements
The addition of other elements in steel, during the steelmaking process, affects its conductivity. While iron provides the base conductivity, the presence of carbon and other alloying elements can influence the overall electrical characteristics. Generally, increasing the carbon content in steel slightly decreases its conductivity. This is because carbon atoms disrupt the regular lattice structure of iron, hindering the free movement of electrons. However, the effect is relatively small, and steel remains a good conductor even with higher carbon content. Other alloying elements also play a role, each influencing the conductivity in a specific way. The exact effect depends on the type and concentration of the alloying elements.
Factors Affecting Steel's Electrical Conductivity
Several factors besides the chemical composition influence the electrical conductivity of steel:
1. Temperature:
Temperature has a significant impact on the conductivity of steel. As temperature increases, the atoms within the steel lattice vibrate more vigorously. This increased atomic vibration interferes with the free movement of electrons, reducing the electrical conductivity. Conversely, lower temperatures lead to less atomic vibration and consequently, higher conductivity. This relationship is generally linear over a certain temperature range.
2. Impurities and Defects:
The presence of impurities and defects in the steel's crystalline structure can significantly affect conductivity. Impurities act as scattering centers for electrons, impeding their flow and decreasing conductivity. Similarly, defects like dislocations and grain boundaries can disrupt the regular lattice structure, leading to increased electron scattering and a decrease in conductivity. High-purity steel generally exhibits higher conductivity than steel with numerous impurities and defects.
3. Physical State:
The physical state of the steel, whether it's in solid, liquid, or gaseous form, dramatically alters its conductivity. Solid steel exhibits considerably higher conductivity than liquid or gaseous steel. In the liquid or gaseous state, the atoms are much more mobile and disordered, hindering the free movement of electrons.
4. Cross-sectional Area and Length:
The physical dimensions of the steel also play a crucial role. A larger cross-sectional area provides more pathways for electrons to flow, increasing conductivity. Conversely, a longer piece of steel offers greater resistance to electron flow, reducing conductivity. This relationship is governed by Ohm's law and is vital in electrical engineering applications involving steel.
Comparing Steel's Conductivity to Other Materials
To put steel's conductivity into perspective, it's helpful to compare it to other common materials:
- Copper: Copper is a significantly better conductor than steel. Its higher conductivity is due to its superior atomic structure and fewer impurities.
- Aluminum: Aluminum also exhibits higher conductivity than steel, making it a popular choice in electrical wiring.
- Silver: Silver possesses the highest electrical conductivity among all metals, but its cost prohibits widespread use.
- Insulators: Materials like rubber, glass, and plastic are insulators. They have tightly bound electrons that cannot move freely, resulting in negligible electrical conductivity.
While steel's conductivity is lower than copper or aluminum, it remains a good conductor and is frequently used in applications where its mechanical strength and other properties are more important than maximizing conductivity.
Practical Implications of Steel's Conductivity
The conductive nature of steel has significant implications across various applications:
1. Electrical Wiring (Limited Use):
While not the preferred choice for high-current applications due to its lower conductivity compared to copper or aluminum, steel is occasionally used in electrical wiring, particularly in situations where its high tensile strength is advantageous.
2. Grounding and Earthing:
Steel's conductivity makes it an excellent material for grounding and earthing systems. Steel rods and conductors are used to safely dissipate electrical current into the earth, protecting against electrical shocks and preventing damage to electrical equipment.
3. Electromagnetic Shielding:
Steel's ability to conduct electricity also makes it an effective material for electromagnetic shielding. Steel enclosures and structures can effectively block electromagnetic radiation, protecting sensitive electronic equipment from interference.
4. Heating Elements:
The electrical resistance of steel, though lower than some other materials, is exploited in some heating elements. The passage of current through a steel wire generates heat due to resistive losses, which is utilized in certain industrial heating applications.
5. Structural Components in Electrical Equipment:
Steel's high strength-to-weight ratio and good conductivity make it suitable for structural components within electrical equipment. While not the primary conductor, it can serve as a supportive structure while also contributing to electrical pathways within a device.
Conclusion: Steel - A Practical Conductor
In conclusion, steel is unequivocally a conductor of electricity, though not as efficient as copper or aluminum. Its conductivity is influenced by factors such as temperature, impurities, and its physical dimensions. While not ideal for all electrical applications requiring maximum conductivity, steel's robust mechanical properties coupled with its decent conductivity make it a versatile material suited for a wide range of applications where both electrical and structural integrity are important considerations. Understanding the nuances of steel's electrical behavior is crucial in designing and implementing various engineering solutions, ensuring safety and optimal performance. The choice of material, therefore, hinges on carefully balancing the trade-off between conductivity and other desired properties.
Latest Posts
Latest Posts
-
Which Of The Following Statements Correctly Describes Gene Linkage
Apr 02, 2025
-
Complete The Complementary Strand Of Dna
Apr 02, 2025
-
Is Carbon Dioxide A Pure Substance Or Mixture
Apr 02, 2025
-
The Given Reaction Proceeds In Two Parts
Apr 02, 2025
-
Which Of The Following Combinations Is Correct
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is Steel A Conductor Or Insulator . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
