Is Sodium Chloride A Homogeneous Mixture
News Leon
Mar 23, 2025 · 5 min read
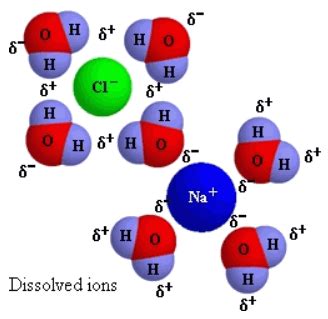
Table of Contents
Is Sodium Chloride a Homogeneous Mixture? A Deep Dive into Chemical Composition and Mixtures
The question, "Is sodium chloride a homogeneous mixture?" might seem simple at first glance. However, understanding the answer requires delving into the fundamental definitions of mixtures, compounds, and the unique properties of sodium chloride (NaCl), commonly known as table salt. This comprehensive article will explore the chemical nature of sodium chloride, differentiate between homogeneous and heterogeneous mixtures, and definitively answer the question while exploring related concepts in chemistry.
Understanding Mixtures and Their Classifications
Before we tackle the specific case of sodium chloride, let's establish a clear understanding of mixtures. A mixture is a substance comprising two or more components not chemically bonded. Crucially, these components retain their individual chemical properties within the mixture. This distinguishes mixtures from chemical compounds, where components undergo a chemical reaction, forming new substances with different properties.
Mixtures are broadly classified into two main types:
Homogeneous Mixtures
A homogeneous mixture exhibits a uniform composition throughout. This means that at a macroscopic level (visible to the naked eye or with standard magnification), the mixture appears as a single phase. The components are evenly distributed, and there are no visually discernible boundaries between them. Examples include saltwater, air, and many alloys.
Heterogeneous Mixtures
In contrast, a heterogeneous mixture has a non-uniform composition. Different components are visibly distinct, and their proportions vary from one region of the mixture to another. Examples include sand and water, oil and water, and a salad. These mixtures often have multiple phases easily identifiable.
The Chemical Composition of Sodium Chloride
Sodium chloride (NaCl) is an ionic compound, not a mixture. This distinction is crucial. It's formed through a chemical reaction between sodium (Na), an alkali metal, and chlorine (Cl), a halogen. During this reaction, sodium atoms lose one electron to chlorine atoms, resulting in the formation of positively charged sodium ions (Na+) and negatively charged chloride ions (Cl−). These ions are held together by strong electrostatic forces of attraction, forming a crystal lattice structure.
The key point here is that sodium and chlorine lose their individual properties when forming sodium chloride. The resulting compound, NaCl, has its own unique set of physical and chemical properties that differ significantly from those of its constituent elements. Sodium is a highly reactive metal, while chlorine is a toxic gas. However, sodium chloride is a relatively inert, crystalline solid, commonly used as a seasoning and preservative.
Why Sodium Chloride is NOT a Homogeneous Mixture
Given the chemical nature of sodium chloride, classifying it as a homogeneous mixture is inaccurate. A homogeneous mixture requires the presence of at least two different substances that retain their individual identities. In NaCl, sodium and chlorine have chemically reacted to form a new substance with a unique identity and properties. There are no separate components in the sense of a homogeneous mixture like saltwater, where both salt and water can be recovered by physical means (e.g., evaporation).
To emphasize this further, consider the following:
- Chemical Bonding: The strong ionic bonds within the NaCl crystal lattice firmly bind the sodium and chloride ions. There's no separation between them on a macroscopic scale.
- Uniformity: At a macroscopic level, a sample of pure sodium chloride appears uniform – a white crystalline solid. However, this uniformity arises from the regular arrangement of ions within the crystal structure, not from a random distribution of different substances.
- Separation of Components: It's impossible to separate sodium and chlorine from sodium chloride through physical methods like filtration or decantation. You need a chemical reaction (electrolysis, for example) to break the ionic bonds and recover the constituent elements.
Common Misconceptions
The confusion surrounding the classification of sodium chloride often stems from the following misconceptions:
- Saltwater vs. Sodium Chloride: Saltwater is a homogeneous mixture of sodium chloride (dissolved) and water. The sodium chloride itself is not the mixture; it's a dissolved component within a mixture.
- Crystalline Structure and Uniformity: The crystal structure of sodium chloride, while uniform at the macroscopic level, doesn't equate to it being a homogeneous mixture. The uniformity arises from the ordered arrangement of ions, not from the mixing of separate substances.
- Salt as a Mixture: The everyday understanding of "salt" often encompasses impure forms containing various trace elements. While these impure forms might be considered heterogeneous mixtures, pure sodium chloride (NaCl) is definitively a compound.
Distinguishing Compounds from Mixtures: A Summary
The critical difference between a compound and a mixture lies in the nature of the bonding involved. In a compound, chemical bonds (ionic, covalent, metallic) create a new substance with distinct properties. The components lose their individual identities. In a mixture, no chemical bonds form; components retain their individual properties and can often be separated by physical means. Sodium chloride falls squarely into the category of a compound because of the strong ionic bonds between sodium and chlorine ions.
Applications and Relevance of Understanding this Distinction
The understanding that sodium chloride is a compound, not a mixture, has significant implications across various scientific fields:
- Chemistry: Accurate classification is essential for understanding chemical reactions, stoichiometry, and material properties.
- Material Science: The crystal structure and properties of sodium chloride are critical for applications such as salt production, food preservation, and industrial processes.
- Biology: Sodium chloride plays a vital role in biological systems, affecting processes like water balance and nerve impulse transmission. Understanding its chemical nature is crucial for physiological studies.
- Geology: Sodium chloride is a significant component of many geological formations, including salt deposits. Its chemical properties influence geological processes.
Conclusion
In conclusion, sodium chloride (NaCl) is not a homogeneous mixture. It's a chemically bonded ionic compound formed from the reaction of sodium and chlorine. While it exhibits uniformity at a macroscopic level, this uniformity results from the ordered arrangement of ions within its crystal structure, not from the mixing of separate substances retaining individual identities. Understanding this crucial distinction between mixtures and compounds is essential for comprehending the fundamental principles of chemistry and their practical applications across various scientific disciplines. The misconception likely stems from the common observation of salt dissolved in water, which constitutes a homogeneous mixture, rather than the chemical nature of pure sodium chloride itself. Therefore, classifying sodium chloride as a mixture is a significant misinterpretation of its chemical composition and bonding.
Latest Posts
Latest Posts
-
What Is The Electron Configuration Of Ni
Mar 25, 2025
-
How Many Hours Is In 3 Weeks
Mar 25, 2025
-
The Y Axis Of A Velocity Time Graph Represents
Mar 25, 2025
-
How Many Moles In 22g Of Co2
Mar 25, 2025
-
A Toiroidal Solenoid Has A Central Radius Of 0 5m
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Is Sodium Chloride A Homogeneous Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
