Is Sodium A Metal Or Nonmetal
News Leon
Mar 20, 2025 · 6 min read
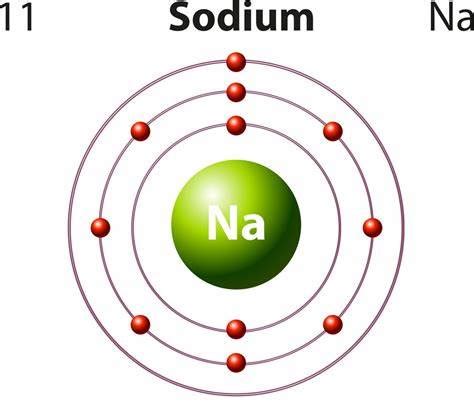
Table of Contents
Is Sodium a Metal or Nonmetal? A Deep Dive into Alkali Metals
Sodium (Na), a ubiquitous element found in table salt (NaCl), often sparks the question: is it a metal or a nonmetal? The answer, unequivocally, is metal. However, understanding why sodium is classified as a metal requires exploring its properties and contrasting them with those of nonmetals. This comprehensive article will delve into the characteristics that definitively place sodium within the metal category, examining its atomic structure, physical properties, chemical reactivity, and its crucial role in various biological and industrial applications.
Understanding the Periodic Table and Metallic Character
Before diving into sodium's specific properties, it's crucial to grasp the broader context of the periodic table. The periodic table organizes elements based on their atomic structure and recurring chemical properties. Elements are broadly categorized into metals, nonmetals, and metalloids (elements exhibiting properties of both metals and nonmetals). Metals, generally located on the left side of the periodic table, share a common set of characteristics. Nonmetals, conversely, reside on the right side and exhibit distinct, contrasting properties.
The metallic character of an element generally increases as you move down and to the left within the periodic table. This trend reflects the increasing ease with which atoms lose electrons to form positive ions (cations). Sodium, being an alkali metal located in the first group and third period of the periodic table, exhibits a strong metallic character.
Sodium's Atomic Structure: The Key to Metallic Behavior
At the heart of sodium's metallic nature lies its atomic structure. A neutral sodium atom possesses 11 electrons arranged in three electron shells: 2 in the inner shell, 8 in the second shell, and a single electron in the outermost shell (valence shell). This lone valence electron is loosely held by the nucleus. This loose electron is the key to understanding sodium's behavior.
The ease with which sodium loses this single valence electron is the cornerstone of its metallic properties. This electron readily participates in metallic bonding, a type of chemical bonding found in metals where valence electrons are delocalized and shared among a lattice of positively charged metal ions. This "sea" of delocalized electrons explains many of sodium's characteristic metallic properties.
Contrasting with Nonmetals: Electron Configuration and Bonding
Nonmetals, on the other hand, tend to have nearly filled valence shells. They achieve stability by gaining electrons, forming negative ions (anions) rather than losing them. This fundamental difference in electron behavior leads to entirely different types of bonding (covalent bonding in nonmetals) and physical properties.
Physical Properties of Sodium: Manifestations of Metallic Character
Numerous physical properties of sodium directly reflect its metallic nature:
1. Electrical Conductivity: A Sea of Mobile Electrons
Sodium is an excellent conductor of electricity. The delocalized electrons in the metallic lattice are free to move throughout the structure. When an electric field is applied, these electrons readily flow, creating an electric current. This high electrical conductivity is a hallmark of metals and sharply contrasts with the poor conductivity of nonmetals.
2. Thermal Conductivity: Efficient Heat Transfer
Sodium is also an excellent conductor of heat. The mobile electrons are not only responsible for electrical conductivity but also facilitate efficient heat transfer throughout the metallic lattice. When heat is applied to one end of a sodium sample, the kinetic energy of the electrons and ions is rapidly transferred throughout the material, leading to rapid temperature equalization. Again, this high thermal conductivity is a key metallic property.
3. Malleability and Ductility: Shaping the Metal
Sodium is highly malleable (can be easily hammered into sheets) and ductile (can be drawn into wires). These properties are directly related to the ability of the metallic lattice to deform without fracturing. The delocalized electrons act as a "glue," holding the positively charged metal ions together even when the lattice is deformed. Nonmetals, with their rigid, localized bonding, lack this property and are generally brittle.
4. Luster: The Shine of a Metal
Sodium possesses a silvery-white metallic luster. This shine arises from the interaction of light with the delocalized electrons in the metal. The electrons absorb and re-emit light of various wavelengths, creating the characteristic metallic sheen. This luster is another visual indicator of metallic character.
5. Density: Relatively Low for a Metal
While sodium is a metal, it's relatively light compared to many other metals like iron or lead. Its low density stems from its relatively large atomic radius and the relatively loose packing of the ions in its lattice structure. This does not, however, negate its metallic properties.
Chemical Properties of Sodium: Reactivity and Ionization
The chemical behavior of sodium is also strongly influenced by its electronic structure. The single valence electron is easily lost, resulting in the formation of a stable +1 ion (Na⁺). This tendency to lose an electron and form a cation is a defining feature of metals.
1. Reactivity with Water: A Vigorous Reaction
Sodium reacts violently with water, producing hydrogen gas and sodium hydroxide (NaOH). The reaction is highly exothermic (releases a significant amount of heat), often igniting the hydrogen gas produced. This high reactivity is indicative of a metal's tendency to readily lose electrons.
2. Reaction with Halogens: Salt Formation
Sodium reacts readily with halogens (e.g., chlorine, bromine, iodine) to form ionic compounds known as salts. For example, the reaction between sodium and chlorine produces sodium chloride (NaCl), common table salt. In these reactions, sodium loses its valence electron to the halogen, forming ionic bonds. The resulting compounds are typically crystalline solids with high melting points.
3. Reduction Reactions: Electron Donation
Sodium acts as a reducing agent in many chemical reactions. This means it readily donates electrons to other atoms or molecules, reducing their oxidation state. This electron donation capability further underscores its metallic character.
Sodium's Importance in Biological and Industrial Applications
The unique properties of sodium make it essential in diverse fields:
Biological Roles: Sodium ions (Na⁺) play a vital role in many biological processes, including nerve impulse transmission, muscle contraction, and fluid balance within the body. Sodium-potassium pumps, crucial for maintaining cellular membrane potential, rely on the movement of sodium ions across cell membranes.
Industrial Applications: Sodium is used extensively in various industries, including:
- Sodium lamps: Sodium vapor lamps produce a bright, yellowish-orange light, making them suitable for street lighting and other applications.
- Sodium hydroxide (NaOH) production: Sodium is a crucial component in the production of sodium hydroxide (lye), a strong base with numerous applications in various industrial processes, including soap manufacturing and paper production.
- Sodium-sulfur batteries: Sodium is used in high-performance batteries due to its high reactivity and ability to readily donate electrons.
Conclusion: Sodium – Undeniably a Metal
In conclusion, sodium's atomic structure, physical properties, and chemical reactivity all definitively point to its classification as a metal. Its tendency to lose its single valence electron, its excellent conductivity (electrical and thermal), its malleability and ductility, and its high reactivity are all hallmarks of metallic character. While its low density might seem to be an exception, it doesn't negate the overwhelming evidence establishing sodium firmly within the realm of metals. Its widespread applications in biology and industry further highlight the importance of this reactive yet crucial metallic element. Therefore, the simple answer to the question, "Is sodium a metal or nonmetal?" is a clear and resounding: metal.
Latest Posts
Latest Posts
-
How Did The Family Reach The Tropical Island
Mar 20, 2025
-
What Kingdom Does A Paramecium Belong To
Mar 20, 2025
-
How Many Hearts Are In A 52 Card Deck
Mar 20, 2025
-
What Makes A Great Leader Essay
Mar 20, 2025
-
What Is The Opposite Of Opaque
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Is Sodium A Metal Or Nonmetal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
