Is Neon A Metal Metalloid Or Nonmetal
News Leon
Mar 28, 2025 · 6 min read
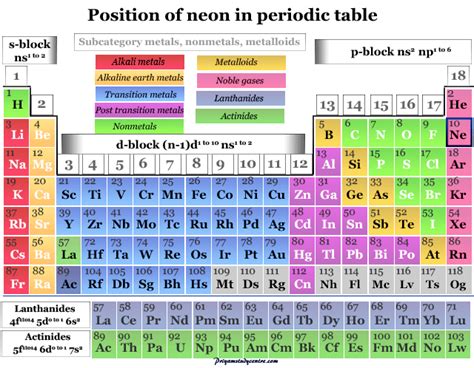
Table of Contents
Is Neon a Metal, Metalloid, or Nonmetal? A Comprehensive Exploration
Neon, a gas that illuminates our world with its vibrant glow, occupies a distinct position on the periodic table. But where exactly does it fall in the classification of elements? Is it a metal, a metalloid, or a nonmetal? This comprehensive exploration will delve deep into neon's properties, explaining its classification and shedding light on the characteristics that define each category.
Understanding the Periodic Table's Organization
The periodic table is a structured arrangement of chemical elements, organized by their atomic number, electron configuration, and recurring chemical properties. Elements are grouped into broad categories: metals, metalloids (or semimetals), and nonmetals. This categorization allows scientists and students to predict an element's behavior based on its location within the table. Understanding these categories is crucial for deciphering the nature of elements like neon.
Metals: A Sea of Shared Electrons
Metals typically reside on the left side of the periodic table. They're characterized by several key properties:
- Excellent electrical and thermal conductivity: Metals readily conduct electricity and heat due to the ease with which electrons move through their structure. This is a direct result of the way metal atoms bond, forming a "sea" of delocalized electrons.
- Malleability and ductility: Metals can be easily hammered into shapes (malleability) and drawn into wires (ductility), a testament to their ability to deform without breaking.
- Luster: Most metals possess a characteristic shine or luster, a result of their interaction with light.
- High density and melting points: Generally, metals have relatively high densities and melting points, reflecting the strong bonds between their atoms.
Examples of metals include iron, copper, gold, and aluminum. These elements clearly exhibit the properties described above.
Metalloids: Bridging the Gap
Metalloids occupy a narrow band between metals and nonmetals on the periodic table. They exhibit properties of both categories, making them unique and versatile. Key characteristics of metalloids include:
- Semiconductor behavior: Metalloids are often semiconductors, meaning their electrical conductivity lies somewhere between metals (good conductors) and nonmetals (insulators). Their conductivity can be altered by temperature or the addition of impurities (doping). This makes them crucial in electronics.
- Variable properties: Their physical and chemical properties can vary significantly depending on conditions.
- Brittle nature: Unlike metals, metalloids tend to be brittle and lack the malleability and ductility seen in metals.
Silicon, germanium, arsenic, and antimony are prime examples of metalloids. Their semiconductor behavior has revolutionized the electronics industry.
Nonmetals: A Diverse Group
Nonmetals are situated on the upper right side of the periodic table. They are characterized by a lack of metallic properties, showcasing a contrasting set of characteristics:
- Poor electrical and thermal conductivity: Nonmetals generally resist the flow of electricity and heat.
- Brittle: Most nonmetals are brittle solids, and some are gases at room temperature.
- Low density and melting points: Nonmetals typically have lower densities and melting points compared to metals.
- Variety of chemical behaviors: Nonmetals display a wide range of chemical behaviors, forming diverse compounds.
Examples of nonmetals include oxygen, nitrogen, chlorine, and sulfur. These elements showcase the features distinctly different from metals.
Neon: A Definitive Nonmetal
Neon, with its atomic number 10, falls squarely within the nonmetal category. Its properties strongly support this classification:
- Gas at room temperature: Neon exists as a colorless, odorless, monatomic gas at standard temperature and pressure. This is a hallmark characteristic of many nonmetals.
- Poor conductor of electricity and heat: Neon, like other nonmetals, is a poor conductor of both electricity and heat. This is directly related to its electronic structure and the strong hold its nucleus has on its electrons.
- Low density and boiling point: Neon has an extremely low density and boiling point, further solidifying its status as a nonmetal.
- Inert nature: Neon is a noble gas, meaning it's exceptionally unreactive due to its complete outer electron shell (octet). This lack of reactivity is a common trait amongst many nonmetals, particularly noble gases. This inertness is a key reason why neon is often used in lighting applications without risk of chemical reactions.
The electronic configuration of neon (1s²2s²2p⁶) is crucial. This complete outermost electron shell (valence shell) indicates exceptional stability and explains its inert nature and lack of metallic properties. Metals, on the other hand, readily lose electrons from their outer shells, leading to their conductive properties and reactivity.
Neon's Use in Lighting and Other Applications
The unique properties of neon make it invaluable in various applications:
- Neon lighting: Its characteristic bright orange-red glow, when subjected to an electrical discharge, is famous and widely used in signage and decorative lighting. This is possible because the electrons in the neon atoms, when energized, release photons of specific wavelengths which corresponds to the characteristic orange-red color.
- Helium-neon lasers: Mixtures with helium are used in lasers to produce coherent light used in various scientific and industrial applications.
- Cryogenics: Neon's low boiling point makes it a useful cryogenic refrigerant in specialized applications requiring extremely low temperatures.
These applications highlight the beneficial properties of neon that stem directly from its nonmetallic characteristics.
Differentiating Neon from Metals and Metalloids
The differences between neon and metals and metalloids are significant and easily observed:
| Feature | Neon (Nonmetal) | Metal | Metalloid |
|---|---|---|---|
| Electrical Conductivity | Poor | Excellent | Semiconductor |
| Thermal Conductivity | Poor | Excellent | Intermediate |
| State at Room Temperature | Gas | Solid (mostly) | Solid |
| Malleability/Ductility | None | High | Low |
| Reactivity | Extremely Low (Inert) | Varies, often high | Varies, intermediate |
| Appearance | Colorless gas | Shiny, lustrous | Variable, often dull |
This table illustrates the clear distinctions between neon and both metals and metalloids. Its properties are completely incompatible with the characteristics of metals, and it lacks the semiconducting behavior typical of metalloids.
Conclusion: Neon's Unwavering Nonmetallic Identity
In conclusion, neon unequivocally belongs to the nonmetal category. Its properties, including its gaseous state at room temperature, poor electrical conductivity, inert nature, and low density, align perfectly with the defining characteristics of nonmetals. Its unique properties make it a valuable element with diverse applications, further solidifying its place as a distinct and important nonmetal. There is no ambiguity; neon is a nonmetal, its position on the periodic table and its physical and chemical behavior clearly confirm this categorization. Its inertness and distinctive glowing properties offer a testament to the fascinating diversity within the nonmetal group of elements. The study of neon, therefore, provides a valuable case study for understanding the broader classification and properties of elements on the periodic table.
Latest Posts
Latest Posts
-
When Sodium Atoms Form Sodium Ions They
Mar 31, 2025
-
Where Is The Starch Stored In Plants
Mar 31, 2025
-
When A Muscle Is Unable To Respond To Stimuli Temporarily
Mar 31, 2025
-
What Is The Empirical Formula For Glucose C6h12o6
Mar 31, 2025
-
Below Is The Structure For The Antibiotic Mycomycin
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Is Neon A Metal Metalloid Or Nonmetal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
