When Sodium Atoms Form Sodium Ions They
News Leon
Mar 31, 2025 · 6 min read
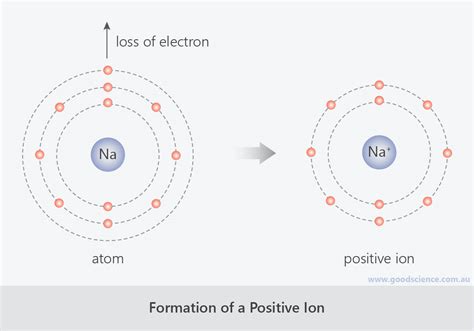
Table of Contents
When Sodium Atoms Form Sodium Ions: A Deep Dive into Ionic Bonding and its Implications
Sodium, a ubiquitous element crucial for life, doesn't exist in its atomic form in nature. Instead, it readily loses an electron to become a positively charged ion, a sodium ion (Na⁺). This transformation, a cornerstone of chemistry, illustrates the fundamental principles of ionic bonding and has profound implications across various scientific fields. This article will explore the process of sodium atom ionization, the driving forces behind it, its consequences, and its relevance in different contexts.
Understanding the Atomic Structure of Sodium
To comprehend the formation of sodium ions, we must first understand the atomic structure of sodium. Sodium (Na), with an atomic number of 11, possesses 11 protons in its nucleus and 11 electrons orbiting the nucleus in specific energy levels or shells. The electron configuration is 2, 8, 1. This means:
- First shell (K-shell): Contains 2 electrons.
- Second shell (L-shell): Contains 8 electrons.
- Third shell (M-shell): Contains only 1 electron.
This lone electron in the outermost shell, known as the valence electron, is relatively loosely bound to the nucleus. This is because it experiences a relatively weak electrostatic attraction from the nucleus, which is shielded by the inner 10 electrons. This characteristic makes the sodium atom highly reactive.
The Ionization Process: Losing an Electron
The formation of a sodium ion from a sodium atom involves the loss of this single valence electron. This process is called ionization, and it results in a stable ion with a complete outer electron shell. The equation representing this process is:
Na → Na⁺ + e⁻
Where:
- Na represents the neutral sodium atom.
- Na⁺ represents the sodium ion (cation) with a +1 charge.
- e⁻ represents the ejected electron.
This ionization process isn't spontaneous in isolation. It requires energy input, known as the ionization energy. The ionization energy for sodium is relatively low compared to other elements, indicating the relative ease with which it loses its valence electron. This low ionization energy is a direct consequence of the shielding effect mentioned earlier and the relatively large distance of the valence electron from the positively charged nucleus.
The Driving Force: Achieving Stability
The driving force behind the ionization of sodium is the quest for stability. Atoms strive to achieve a stable electron configuration, most often resembling that of a noble gas. Noble gases, such as neon (Ne), have a full outermost electron shell, making them exceptionally unreactive. By losing its single valence electron, sodium achieves an electron configuration identical to that of neon (2, 8), a stable octet. This stable configuration minimizes the atom’s energy, making it more stable and less reactive.
The Role of Electronegativity
Electronegativity plays a significant role in the formation of sodium ions. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. Sodium has a low electronegativity, meaning it has a weak ability to attract electrons. When sodium encounters an element with higher electronegativity, such as chlorine (Cl), the electronegativity difference drives the electron transfer. Chlorine, with a strong attraction for electrons, readily accepts the electron lost by sodium.
Ionic Bonding: The Formation of Sodium Chloride
The ionization of sodium and the subsequent acceptance of the electron by another atom often leads to the formation of an ionic bond. This is a type of chemical bond formed through the electrostatic attraction between oppositely charged ions. A classic example is the formation of sodium chloride (NaCl), common table salt. The reaction is:
2Na + Cl₂ → 2NaCl
In this reaction, each sodium atom loses one electron to become a Na⁺ ion, while each chlorine atom gains one electron to become a Cl⁻ ion. The resulting electrostatic attraction between the positively charged sodium ions and the negatively charged chloride ions forms the ionic compound, sodium chloride. This is a crystal lattice structure where Na⁺ and Cl⁻ ions are arranged in a regular, repeating pattern.
Properties of Sodium Ions and their Compounds
The formation of sodium ions significantly alters the properties of sodium. Sodium metal is a soft, silvery-white, highly reactive solid. However, sodium ions, being part of an ionic compound, exhibit different properties:
- Solubility: Many sodium compounds are soluble in water, due to the strong interaction between water molecules and the charged ions.
- Conductivity: Molten sodium chloride or aqueous solutions of sodium chloride conduct electricity because the mobile ions can carry an electric current.
- Melting and Boiling Points: Ionic compounds like sodium chloride have high melting and boiling points because of the strong electrostatic forces between the ions.
Biological Significance of Sodium Ions
Sodium ions play a vital role in various biological processes:
- Nerve Impulse Transmission: Sodium ions are crucial for the transmission of nerve impulses. The movement of sodium ions across cell membranes generates the electrical signals that allow nerve cells to communicate.
- Muscle Contraction: Sodium ions are essential for muscle contraction. Changes in sodium ion concentrations trigger the muscle fibers to contract.
- Fluid Balance: Sodium ions help regulate fluid balance in the body. They maintain the osmotic pressure of body fluids, preventing excessive fluid loss or retention.
- Nutrient Absorption: Sodium ions are involved in the absorption of nutrients from the digestive tract.
Sodium imbalances can lead to serious health issues, emphasizing the crucial role of sodium ions in maintaining homeostasis.
Industrial Applications
Sodium compounds have numerous industrial applications, largely due to the properties conferred by the presence of sodium ions:
- Production of Sodium Hydroxide (NaOH): Sodium hydroxide, a strong alkali, is widely used in various industries, including soap making, paper production, and water treatment.
- Sodium Chloride in Food Preservation: Sodium chloride is used as a preservative in many food products due to its ability to inhibit bacterial growth.
- Sodium Lamps: High-intensity sodium lamps are used in street lighting due to their high efficiency and bright yellow light.
- Sodium in Chemical Synthesis: Sodium metal and its compounds are used as reagents in various chemical syntheses.
Environmental Considerations
While sodium ions are essential for life, excessive amounts in the environment can have negative consequences. For example, high levels of sodium in water bodies can affect aquatic life, leading to salinity issues and harming sensitive species. Furthermore, the overuse of sodium-containing fertilizers can contribute to soil salinization, degrading agricultural land.
Conclusion
The transformation of a sodium atom into a sodium ion is a fundamental chemical process that illustrates the principles of ionic bonding and the quest for atomic stability. The loss of a single valence electron leads to significant changes in the physical and chemical properties of sodium, resulting in the formation of stable ionic compounds with a wide range of applications in biology and industry. Understanding this process is crucial in various scientific fields and highlights the importance of considering the environmental impact of sodium compounds and their responsible use. The relatively low ionization energy of sodium, coupled with its high reactivity, has made it a pivotal element in many areas of science and technology, underscoring the fundamental importance of this seemingly simple ionic transformation. Future research continues to explore the intricacies of sodium ion behavior in diverse environments, from biological systems to industrial processes, promising further advancements in various scientific disciplines.
Latest Posts
Latest Posts
-
Greatest Common Factor Of 36 And 20
Apr 02, 2025
-
What Is The Antonym Of Urban
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about When Sodium Atoms Form Sodium Ions They . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
