Is Lioh An Acid Or Base
News Leon
Mar 22, 2025 · 5 min read
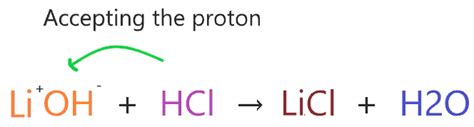
Table of Contents
- Is Lioh An Acid Or Base
- Table of Contents
- Is LiOH an Acid or Base? Understanding the Properties of Lithium Hydroxide
- Understanding Acids and Bases: A Quick Recap
- Arrhenius Theory:
- Brønsted-Lowry Theory:
- LiOH: A Strong Base Unveiled
- Why is complete dissociation crucial?
- LiOH's Properties and Characteristics
- Applications of Lithium Hydroxide: A Versatile Compound
- 1. Ceramic Industry:
- 2. Lubricants:
- 3. Alkaline Batteries:
- 4. Chemical Synthesis:
- 5. Carbon Dioxide Scrubbing:
- 6. Nuclear Reactors:
- 7. Organic Chemistry:
- Safety Precautions: Handling LiOH Responsibly
- Distinguishing LiOH from Other Compounds
- Conclusion: LiOH – A Strong Base with Diverse Applications
- Latest Posts
- Latest Posts
- Related Post
Is LiOH an Acid or Base? Understanding the Properties of Lithium Hydroxide
Lithium hydroxide (LiOH), a seemingly simple chemical compound, often sparks confusion regarding its acidic or basic nature. This comprehensive guide will delve deep into the properties of LiOH, explaining why it's unequivocally classified as a strong base, and exploring its various applications, reactions, and safety considerations. We'll unravel the complexities behind its behavior, providing a clear understanding for both beginners and those with a more advanced chemistry background.
Understanding Acids and Bases: A Quick Recap
Before we dive into the specifics of LiOH, let's refresh our understanding of acids and bases. Several theories define these fundamental concepts, but the most relevant for our discussion are the Arrhenius and Brønsted-Lowry theories.
Arrhenius Theory:
The Arrhenius theory, one of the earliest models, defines acids as substances that produce hydrogen ions (H⁺) when dissolved in water, and bases as substances that produce hydroxide ions (OH⁻) when dissolved in water.
Brønsted-Lowry Theory:
The Brønsted-Lowry theory offers a broader definition. It defines acids as proton donors (donating H⁺ ions) and bases as proton acceptors. This theory extends the definition beyond aqueous solutions, encompassing reactions in other solvents or even without a solvent.
LiOH: A Strong Base Unveiled
Lithium hydroxide, LiOH, fits squarely within the definition of a strong base, according to both the Arrhenius and Brønsted-Lowry theories. Let's examine why:
When LiOH dissolves in water, it completely dissociates into its constituent ions:
LiOH(s) → Li⁺(aq) + OH⁻(aq)
This complete dissociation is the hallmark of a strong base. The presence of a significant concentration of hydroxide ions (OH⁻) in the solution drastically increases its pH, making it highly alkaline. The pH of a LiOH solution will be significantly greater than 7, indicating its strong basic nature.
Why is complete dissociation crucial?
The complete dissociation of LiOH is crucial in classifying it as a strong base. Weak bases, on the other hand, only partially dissociate in water, resulting in a lower concentration of OH⁻ ions and a less alkaline solution. This partial dissociation is a key differentiator between strong and weak bases.
LiOH's Properties and Characteristics
LiOH possesses several key properties that contribute to its classification as a strong base and its various applications:
-
High Solubility: LiOH is relatively soluble in water, unlike some other hydroxides. This high solubility ensures that a significant amount of OH⁻ ions are released into the solution, enhancing its basicity.
-
Strong alkalinity: As mentioned previously, the high concentration of OH⁻ ions leads to a highly alkaline solution with a high pH.
-
Hygroscopic nature: LiOH is hygroscopic, meaning it readily absorbs moisture from the atmosphere. This property requires careful storage to prevent its deterioration.
-
Reactivity: LiOH reacts vigorously with acids, neutralizing them in an exothermic reaction (releasing heat). This reaction is a fundamental characteristic of bases.
-
Toxicity: LiOH is toxic and corrosive. Appropriate safety measures must be taken when handling it.
Applications of Lithium Hydroxide: A Versatile Compound
The unique properties of LiOH make it a versatile compound with numerous applications across various industries:
1. Ceramic Industry:
LiOH serves as a valuable raw material in the ceramic industry, enhancing the properties of ceramic materials. It plays a role in controlling the melting point, viscosity, and other important physical characteristics of ceramic products.
2. Lubricants:
Lithium hydroxide finds application in the production of certain types of lubricating greases. Its inclusion contributes to improved lubrication performance under specific conditions.
3. Alkaline Batteries:
LiOH is used in some types of alkaline batteries as an electrolyte. It facilitates the flow of ions and enables the electrochemical reactions within the battery.
4. Chemical Synthesis:
LiOH plays a crucial role as a reactant and catalyst in various chemical syntheses. Its strong basicity makes it a valuable reagent in organic and inorganic chemistry.
5. Carbon Dioxide Scrubbing:
One of the most critical applications of LiOH is its use in carbon dioxide (CO₂) scrubbing. This is particularly relevant in spacecraft and submarines, where maintaining a breathable atmosphere is vital. The reaction between LiOH and CO₂ is as follows:
2LiOH(s) + CO₂(g) → Li₂CO₃(s) + H₂O(l)
This reaction effectively removes CO₂ from the air, ensuring the air's quality remains suitable for respiration.
6. Nuclear Reactors:
LiOH, specifically Lithium-6 Hydroxide (⁶LiOH), plays a significant role in the control and moderation of nuclear reactions. Lithium-6 has a high neutron absorption cross-section, meaning it effectively absorbs neutrons, thus helping to regulate the rate of the nuclear chain reaction.
7. Organic Chemistry:
In organic chemistry, LiOH's strong basicity enables it to participate in various reactions. For example, it can be used in the deprotonation of acidic compounds, or in the hydrolysis of esters.
Safety Precautions: Handling LiOH Responsibly
Given LiOH's corrosive and toxic nature, appropriate safety measures are crucial when handling it:
-
Eye Protection: Always wear safety goggles or a face shield to protect your eyes from splashes.
-
Gloves: Use chemical-resistant gloves to prevent skin contact.
-
Ventilation: Ensure adequate ventilation to avoid inhaling LiOH dust or fumes.
-
Emergency Procedures: Have access to an eyewash station and safety shower in case of accidental exposure.
-
Storage: Store LiOH in a tightly sealed container in a dry, cool place, away from incompatible substances.
Distinguishing LiOH from Other Compounds
It's important to differentiate LiOH from other lithium compounds and bases. While other lithium compounds might have some basic properties, LiOH is unique due to its complete dissociation in water and its high solubility. Furthermore, the strength of the base is significantly influenced by the cation involved. The smaller size and higher charge density of the lithium cation compared to other alkali metals result in stronger base character for LiOH.
Conclusion: LiOH – A Strong Base with Diverse Applications
In conclusion, lithium hydroxide (LiOH) is undoubtedly a strong base. Its complete dissociation in water, high solubility, and high reactivity with acids definitively categorize it as such. This strong basic nature, coupled with its other properties, makes LiOH a valuable compound with diverse applications in various industries, ranging from ceramics and lubricants to carbon dioxide scrubbing and nuclear reactors. However, it’s imperative to remember its toxic and corrosive nature and to always handle it with appropriate safety measures. Understanding its properties and applications provides a solid foundation for utilizing this powerful chemical responsibly and effectively.
Latest Posts
Latest Posts
-
Which Of The Following Is Not A Function Of Neurons
Mar 24, 2025
-
What Does Positive Delta H Mean
Mar 24, 2025
-
State Whether The Following Statements Are True Or False
Mar 24, 2025
-
In Which Stage Of Meiosis Crossing Over Takes Place
Mar 24, 2025
-
Which Of The Following Is Not A Major Nutrient
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Is Lioh An Acid Or Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
