Is Hydrogen A Element Or Compound
News Leon
Mar 22, 2025 · 5 min read
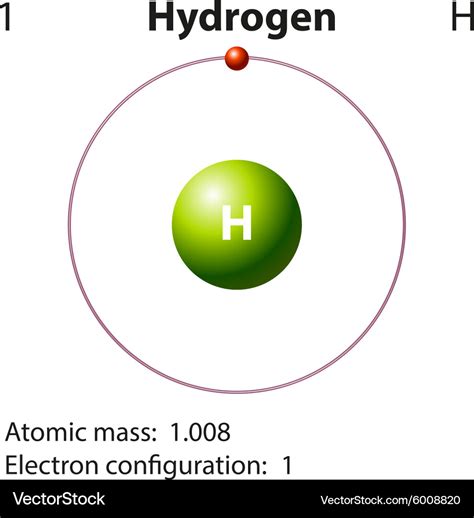
Table of Contents
Is Hydrogen an Element or a Compound? A Deep Dive into Atomic Structure and Molecular Bonds
The question, "Is hydrogen an element or a compound?" might seem simple at first glance. However, delving into the specifics reveals a fascinating exploration of atomic structure, molecular bonding, and the fundamental building blocks of matter. The answer, while seemingly straightforward, requires a nuanced understanding of chemical definitions and the behavior of hydrogen in different contexts.
Understanding the Definitions: Element vs. Compound
Before we delve into the specifics of hydrogen, let's clarify the definitions of element and compound:
-
Element: An element is a pure substance consisting only of atoms that all have the same number of protons in their atomic nuclei. This number is known as the atomic number and uniquely identifies each element. Elements cannot be broken down into simpler substances by chemical means. Examples include oxygen (O), iron (Fe), and gold (Au).
-
Compound: A compound is a pure substance formed when two or more different elements are chemically bonded together in a fixed ratio. These bonds involve the sharing or transfer of electrons between atoms. Compounds can be broken down into their constituent elements through chemical reactions. Examples include water (H₂O), carbon dioxide (CO₂), and table salt (NaCl).
Hydrogen: The Simplest Element
Hydrogen (H), with an atomic number of 1, is the simplest element in the periodic table. It possesses a single proton in its nucleus and, in its most common form, a single electron orbiting that proton. This simplicity is key to understanding its unique properties and why the question of whether it's an element or a compound is even posed.
The Lone Proton: The single proton in the hydrogen atom's nucleus is the defining characteristic that classifies it as an element. This proton dictates its chemical behavior and its place on the periodic table.
Isotopes of Hydrogen: It's crucial to note that hydrogen exists in three isotopes: protium (¹H), deuterium (²H or D), and tritium (³H or T). These isotopes differ in the number of neutrons in their nuclei, but they all retain the defining characteristic of one proton, thus remaining the same element.
Why the Confusion? Diatomic Hydrogen (H₂)
While hydrogen is unequivocally an element, the confusion often arises from its tendency to exist as a diatomic molecule, H₂. This means that two hydrogen atoms readily bond together to form a stable molecule. This bonding is a covalent bond, where the two hydrogen atoms share their single electrons to achieve a more stable electron configuration (similar to that of helium).
Covalent Bonding: The shared electron pair creates a strong attractive force between the two hydrogen atoms, forming the H₂ molecule. This doesn't change the fact that hydrogen is an element; it simply reflects its behavior in its most common natural state.
Misinterpretation: The existence of H₂ molecules can lead to the misconception that hydrogen is a compound because it appears to consist of two atoms. However, these two atoms are of the same element. This is a crucial distinction – a compound requires different elements bonded together.
Hydrogen's Role in Compounds
Although hydrogen itself is an element, it plays a vital role in numerous compounds. Its ability to bond with many other elements contributes to its ubiquitous presence in nature and its importance in chemical processes.
Water (H₂O): Arguably the most important compound containing hydrogen, water is essential for life as we know it. The covalent bonds between hydrogen and oxygen atoms create a unique molecule with remarkable properties.
Hydrocarbons: Hydrogen forms the backbone of numerous hydrocarbons, which are compounds containing only carbon and hydrogen. These compounds are crucial components of fossil fuels (like methane, CH₄) and are used extensively in various industries.
Acids and Bases: Hydrogen plays a central role in defining acids and bases. In the Brønsted-Lowry definition, acids are hydrogen ion (H⁺) donors, while bases are hydrogen ion acceptors.
Hydrogenation Reactions: In organic chemistry, hydrogenation is a crucial process involving the addition of hydrogen atoms to unsaturated compounds (those containing double or triple bonds), often in the presence of a catalyst. This process is widely used in the food and petroleum industries.
Hydrogen's Unique Properties and its Element Status
Several properties further solidify hydrogen's classification as an element:
- Atomic Number: Its atomic number of 1 is unchanging and uniquely identifies it.
- Spectral Lines: Hydrogen has a characteristic emission spectrum, a unique fingerprint of light emitted by its excited atoms. This spectrum is a distinct property of the element, independent of its molecular form.
- Chemical Reactivity: While it readily forms H₂, its reactivity with other elements, forming diverse compounds, demonstrates its elemental nature. The reactions are driven by its single electron seeking stability.
- Physical Properties: The physical properties of hydrogen, such as its low density and boiling point, are inherent properties of the element itself. The properties of H₂ are a consequence of the element's properties and the nature of its diatomic bonding.
Beyond Diatomic Hydrogen: Other Forms of Hydrogen
While H₂ is the most prevalent form of hydrogen, other forms exist, emphasizing its elemental nature:
- Atomic Hydrogen: Under specific conditions, hydrogen can exist as individual atoms, devoid of any chemical bonds. This is relatively rare but highlights the fundamental elemental nature of individual hydrogen atoms.
- Hydrogen Ions (H⁺): In aqueous solutions, hydrogen frequently loses its electron, existing as a proton (H⁺), a positively charged ion. This is an ion, not a molecule or a compound. Again, it remains the same element.
- Metallic Hydrogen: Under extreme pressure, hydrogen is predicted to transition into a metallic state, exhibiting properties characteristic of metals. This theoretical state further reinforces that the underlying substance remains the elemental hydrogen, merely under altered conditions.
Conclusion: Hydrogen Remains an Element
In conclusion, hydrogen is unequivocally an element. While it readily forms diatomic molecules (H₂) and participates in countless compounds, its fundamental atomic structure—a single proton and (usually) a single electron—defines it as an element. The formation of H₂ molecules is a consequence of the element's inherent properties and drive towards chemical stability, not a transformation into a compound. The existence of various forms, from atomic hydrogen to metallic hydrogen, only emphasizes the versatility and remarkable properties of this simplest of elements. The confusion stems from a misunderstanding of the difference between an element and the molecules it can form, a key concept in understanding basic chemistry.
Latest Posts
Latest Posts
-
Which Is A Similarity Between Alcohol Fermentation And Aerobic Respiration
Mar 23, 2025
-
Integrate 1 X 2 X 1
Mar 23, 2025
-
What Is The Mass Of One Mole Of Water
Mar 23, 2025
-
When Hydrogen Atoms Combine To Form Helium
Mar 23, 2025
-
What Is The Atomic Number Of An Atom Equivalent To
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Is Hydrogen A Element Or Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
