Is Density A Physical Or Chemical Change
News Leon
Mar 16, 2025 · 5 min read
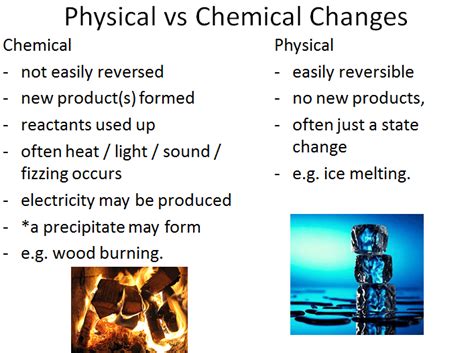
Table of Contents
Is Density a Physical or Chemical Change? Understanding the Fundamentals
Density, a fundamental property of matter, often sparks confusion regarding its classification as a physical or chemical change. This comprehensive guide will delve into the intricacies of density, differentiating physical and chemical changes, and definitively answering the question: Is density a physical or chemical change? We will explore various examples and address common misconceptions, providing a clear understanding of this crucial concept.
What is Density?
Density is defined as the mass of a substance per unit volume. It's a measure of how much matter is packed into a given space. The formula for density is:
Density = Mass / Volume
Density is typically expressed in units like grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³). Understanding density is crucial across numerous scientific disciplines, from material science and engineering to chemistry and physics.
Density as an Intensive Property
It's vital to understand that density is an intensive property. This means that the density of a substance remains constant regardless of the amount of the substance present. A kilogram of gold will have the same density as a gram of gold. This contrasts with extensive properties, such as mass or volume, which change with the amount of substance.
Physical vs. Chemical Changes: A Crucial Distinction
Before we definitively classify density, we must clearly understand the difference between physical and chemical changes.
Physical Changes
A physical change alters the form or appearance of a substance but does not change its chemical composition. Examples include:
- Changes in state: Melting ice (solid to liquid), boiling water (liquid to gas), freezing water (liquid to solid), and sublimation (solid to gas). These changes only affect the arrangement of molecules, not the molecules themselves.
- Changes in shape: Cutting paper, bending a wire, crushing a can. These changes alter the physical form without altering the chemical makeup.
- Dissolving: Dissolving sugar in water. The sugar molecules are dispersed in the water, but their chemical structure remains unchanged. They can be recovered by evaporating the water.
- Mixing: Combining sand and salt. The individual components retain their chemical identities.
Chemical Changes
A chemical change, also known as a chemical reaction, involves the rearrangement of atoms and molecules, resulting in the formation of new substances with different chemical properties. Key indicators of a chemical change include:
- Formation of a gas: Bubbles forming during a reaction.
- Formation of a precipitate: The appearance of a solid from a solution.
- Color change: A significant change in the color of a substance.
- Temperature change: A noticeable increase or decrease in temperature.
- Light emission: The production of light during a reaction.
Density: A Physical Property, Not a Change
Now, we can definitively answer the central question. Density is a physical property, not a physical or chemical change. Measuring density does not alter the substance's chemical composition. You are simply quantifying an inherent characteristic of the material.
Let's illustrate this with examples:
- Determining the density of water: Measuring the mass and volume of a water sample to calculate its density does not change the water's chemical formula (H₂O). The water remains water.
- Comparing the densities of different metals: Determining and comparing the densities of gold, silver, and iron involves measuring mass and volume but does not transform the metals into different substances. They retain their individual chemical identities.
- Calculating the density of a gas: Determining the density of a gas like oxygen involves measuring its mass and volume under specific conditions, but the oxygen remains oxygen.
Misconceptions about Density and Change
Some misconceptions frequently arise concerning density:
- Compression and Density: Compressing a substance, like squeezing a sponge, reduces its volume, thereby increasing its density. However, this is still a physical change. The chemical composition of the sponge remains unchanged.
- Temperature and Density: Temperature changes can affect the density of a substance. For example, heating water generally decreases its density (water is an exception to this rule near its freezing point). Again, this is a physical change, as the water molecules remain H₂O. The change in density is due to altered molecular spacing.
- Density and Chemical Reactions: While density is a physical property, it can be used to infer the outcome of a chemical reaction. For example, if a reaction produces a gas, the decrease in density of the reaction mixture indicates gas formation. However, the density measurement itself isn't the chemical change; it's a consequence of it.
Practical Applications of Density
Density's importance extends far beyond theoretical considerations. Its practical applications are numerous and significant:
- Material Identification: Density is a crucial tool for identifying unknown materials. Comparing the measured density of a substance to known densities of various materials can aid in identification.
- Archimedes' Principle and Buoyancy: Density plays a pivotal role in understanding buoyancy and flotation. Objects less dense than a fluid will float, while denser objects will sink. This principle is fundamental in naval architecture, submarine design, and many other applications.
- Gemology: The density of gemstones is used to authenticate and identify various precious stones. This is a vital aspect of gem grading and quality control.
- Medicine: Density measurements are used in various medical applications, such as bone density scans (osteodensitometry), which assesses the risk of osteoporosis.
Conclusion: Density – An Inherent Physical Property
In conclusion, density is unequivocally a physical property of matter. Measuring or calculating density does not involve a chemical change; it simply quantifies the mass-to-volume ratio of a substance. While external factors like temperature and pressure can affect density, these influences represent physical changes, not chemical transformations. Understanding this distinction is crucial for comprehending the fundamental properties of matter and their various applications in numerous fields. The next time you encounter a question about density and change, remember that it's a physical property and that measuring it doesn't alter the substance's inherent chemical composition.
Latest Posts
Latest Posts
-
Is Boiling Water A Physical Change
Mar 17, 2025
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
-
Which Is The Correct Order Of The Scientific Method
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is Density A Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
