Is Bh3 A Lewis Acid Or Base
News Leon
Mar 24, 2025 · 5 min read
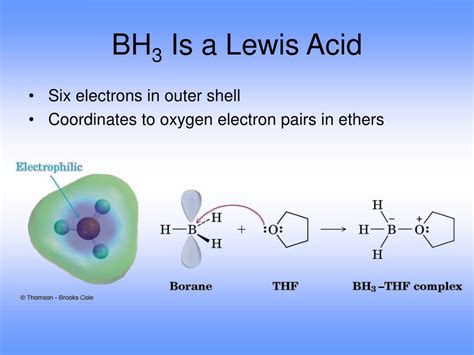
Table of Contents
Is BH₃ a Lewis Acid or Base? Understanding Boron's Role in Chemical Bonding
Boron, a metalloid residing in Group 13 of the periodic table, displays unique chemical behavior, often defying simple categorization. One area of particular interest revolves around its role in Lewis acid-base chemistry. This article delves deep into the question: Is BH₃ a Lewis acid or base? We will explore the concept of Lewis acidity and basicity, examine the electronic structure of BH₃, and analyze its reactivity to provide a comprehensive answer.
Understanding Lewis Acidity and Basicity
Before we can classify BH₃, it's crucial to understand the definitions of Lewis acids and bases. Unlike Brønsted-Lowry definitions focusing on proton transfer, Lewis theory defines acids and bases based on electron pair donation and acceptance.
-
Lewis Acid: A Lewis acid is an electron pair acceptor. It possesses an incomplete octet or has a vacant orbital capable of accepting a lone pair of electrons from a Lewis base.
-
Lewis Base: A Lewis base is an electron pair donor. It contains at least one lone pair of electrons that can be donated to form a coordinate covalent bond with a Lewis acid.
Think of it like this: a Lewis acid is "electron-hungry," while a Lewis base is "electron-rich." The interaction between them forms a coordinate covalent bond, where both electrons in the bond originate from the Lewis base.
The Electronic Structure of BH₃
Boron, having three valence electrons, forms three covalent bonds in BH₃. Each hydrogen atom contributes one electron, resulting in a total of six valence electrons surrounding the boron atom. This arrangement leaves boron with only six electrons in its valence shell, two electrons short of a stable octet. This incomplete octet is the key to understanding BH₃'s Lewis acid behavior.
Orbital Hybridization in BH₃
The boron atom in BH₃ undergoes sp² hybridization. This means that one s orbital and two p orbitals combine to form three sp² hybrid orbitals, which are oriented at 120° angles to each other. These sp² hybrid orbitals participate in the formation of sigma bonds with the three hydrogen atoms. The remaining unhybridized p orbital on boron is empty, making it highly receptive to accepting an electron pair. This empty p orbital is crucial for BH₃'s Lewis acidity.
BH₃'s Reactivity: Evidence of Lewis Acidity
The incomplete octet and the presence of an empty p orbital strongly suggest that BH₃ is a Lewis acid. Its reactivity further confirms this:
-
Dimerization: BH₃ readily dimerizes to form diborane (B₂H₆). In this reaction, two BH₃ molecules share two hydrogen atoms to achieve a more stable configuration. Each boron atom forms four bonds, although it's still electron-deficient. This dimerization is a direct consequence of BH₃'s strong tendency to gain electrons to complete its octet.
-
Reaction with Lewis Bases: BH₃ readily reacts with Lewis bases such as ammonia (NH₃), amines, and phosphines. The lone pair of electrons on the nitrogen or phosphorus atom readily donates to the empty p orbital on boron, forming a coordinate covalent bond. For example, the reaction with ammonia produces BH₃-NH₃, a stable adduct. This facile reaction with Lewis bases is a hallmark of Lewis acids.
-
Hydroboration Reactions: BH₃, often used in the form of its borane complexes like borane-tetrahydrofuran (BH₃·THF), is a crucial reagent in hydroboration reactions. These reactions involve the addition of BH₃ across carbon-carbon double bonds (alkenes). The reaction proceeds through the initial coordination of the alkene's pi electrons to the boron atom, a classic example of Lewis acid-base interaction.
Why BH₃ is NOT a Lewis Base
While BH₃ is electron-deficient, it does not possess a lone pair of electrons that it can donate. This is the fundamental requirement for a Lewis base. The electrons in the B-H bonds are shared equally (or nearly equally) between boron and hydrogen, and are unavailable for donation. Therefore, BH₃ cannot act as a Lewis base.
Comparing BH₃ with other Boron Compounds
Let's contrast BH₃ with other boron compounds to further solidify its classification as a Lewis acid.
-
BF₃: Boron trifluoride (BF₃) is another classic example of a Lewis acid. Similar to BH₃, it has an incomplete octet and an empty p orbital readily available for electron pair acceptance. Its strong Lewis acidity is frequently exploited in organic chemistry.
-
BCl₃: Boron trichloride (BCl₃) also exhibits Lewis acidity, although its strength is somewhat less than that of BF₃ due to the higher electronegativity of fluorine compared to chlorine.
-
B(OH)₃: Boric acid, B(OH)₃, is a slightly weaker Lewis acid compared to BH₃ or BF₃. It accepts a lone pair from water molecules in solution to form tetrahedral species. This indicates a weak Lewis acidic behavior.
Applications of BH₃'s Lewis Acidity
The Lewis acidity of BH₃ and its derivatives finds numerous applications in various fields:
-
Organic Synthesis: Borane reagents are essential in organic synthesis, particularly in hydroboration reactions, which are used to synthesize alcohols and other functional groups from alkenes.
-
Polymer Chemistry: Borane-based catalysts are employed in polymerization reactions, demonstrating their role in initiating and controlling polymer chain growth.
-
Materials Science: Boron compounds find applications in materials science, often as precursors for the synthesis of novel materials with interesting properties. The Lewis acidity of the boron centers plays a role in the self-assembly and reactivity of these materials.
-
Medicine and Biochemistry: While less direct, the understanding of boron's Lewis acidity contributes to the study of interactions between boron-containing compounds and biological systems, opening possibilities for drug discovery and biomedical applications.
Conclusion: BH₃ as a Definitive Lewis Acid
In conclusion, based on its electronic structure, reactivity, and comparison with other boron compounds, BH₃ is unequivocally classified as a Lewis acid. Its incomplete octet and the presence of an empty p orbital make it a strong electron-pair acceptor, readily reacting with Lewis bases to form stable adducts. While it is electron-deficient, it lacks the crucial characteristic of a Lewis base: a lone pair of electrons available for donation. Understanding the Lewis acidity of BH₃ is crucial for comprehending its diverse chemical reactivity and its wide-ranging applications across various scientific and technological domains. Its ability to accept electron pairs drives its importance in numerous synthetic and industrial processes.
Latest Posts
Latest Posts
-
A Convex Lens Of Focal Length 10cm
Mar 26, 2025
-
Gases Have Indefinite Shape And Volume
Mar 26, 2025
-
Round 64 To The Nearest Ten
Mar 26, 2025
-
Which Of The Following Are Meso Compounds
Mar 26, 2025
-
Words Beginning With The Same Letter
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Is Bh3 A Lewis Acid Or Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
