Is Ammonia An Element Compound Or Mixture
News Leon
Mar 15, 2025 · 6 min read
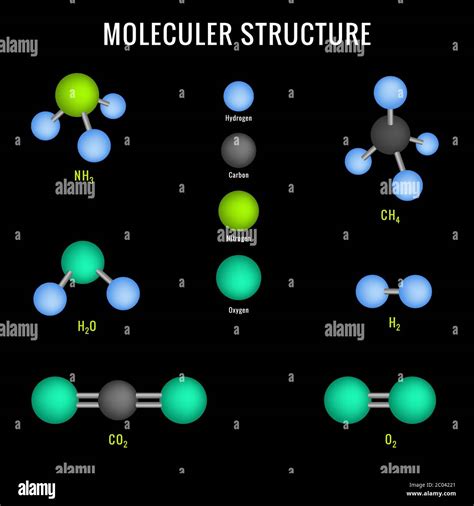
Table of Contents
Is Ammonia an Element, Compound, or Mixture? A Deep Dive into its Chemical Nature
Ammonia, a ubiquitous chemical with a pungent odor, often sparks curiosity regarding its fundamental nature. Is it an element, a compound, or a mixture? Understanding this requires delving into the core concepts of chemistry and exploring ammonia's unique properties and composition. This comprehensive guide will clarify its classification, explore its molecular structure, discuss its properties, and examine its widespread applications.
Understanding the Basic Classifications: Element, Compound, and Mixture
Before we determine the classification of ammonia, let's review the fundamental differences between elements, compounds, and mixtures:
Elements: The Building Blocks of Matter
Elements are pure substances consisting of only one type of atom. These atoms cannot be broken down into simpler substances by chemical means. The periodic table organizes all known elements, each represented by a unique symbol (e.g., H for hydrogen, O for oxygen, N for nitrogen). Examples include gold (Au), oxygen (O), and iron (Fe).
Compounds: A Union of Elements
Compounds are pure substances formed when two or more elements chemically combine in a fixed ratio. This chemical bonding creates a new substance with properties distinct from its constituent elements. The elements in a compound are combined in a definite proportion, which can be represented by a chemical formula (e.g., H₂O for water, NaCl for sodium chloride). The properties of a compound are different from the properties of the elements it is made of. For example, sodium (a highly reactive metal) and chlorine (a toxic gas) combine to form sodium chloride (table salt), a stable and edible compound.
Mixtures: A Blend of Substances
Mixtures are physical combinations of two or more substances, where each substance retains its individual properties. Unlike compounds, mixtures don't have a fixed composition, and their components can be separated by physical means (e.g., filtration, distillation). Examples include air (a mixture of gases), saltwater (a mixture of salt and water), and soil (a mixture of minerals and organic matter).
Ammonia: A Definitive Compound
Now, let's focus on ammonia (NH₃). Ammonia is a compound, not an element or a mixture. This is because:
-
Fixed Composition: Ammonia always contains nitrogen and hydrogen in a specific ratio: one nitrogen atom for every three hydrogen atoms. This fixed ratio is a hallmark of a compound. You cannot have ammonia with varying proportions of nitrogen and hydrogen; it would no longer be ammonia.
-
Chemical Bonding: Nitrogen and hydrogen atoms are held together by strong covalent bonds within the ammonia molecule. Covalent bonds involve the sharing of electrons between atoms, resulting in a stable molecule with distinct properties. This chemical bonding differentiates compounds from mixtures where substances are merely physically mixed.
-
Distinct Properties: Ammonia possesses unique properties that differ significantly from its constituent elements. Nitrogen is an inert gas, while hydrogen is highly flammable. Ammonia, however, is a colorless gas with a characteristic pungent odor and is soluble in water. This change in properties upon chemical combination further confirms its status as a compound.
The Molecular Structure of Ammonia
Understanding the molecular structure of ammonia clarifies its chemical behavior and properties. The ammonia molecule (NH₃) has a trigonal pyramidal geometry. This means:
-
Central Nitrogen Atom: A nitrogen atom sits at the center of the molecule.
-
Three Hydrogen Atoms: Three hydrogen atoms are bonded to the central nitrogen atom.
-
Lone Pair of Electrons: The nitrogen atom also possesses a lone pair of electrons, which contribute to the molecule's polarity and reactivity.
This arrangement gives the ammonia molecule a dipole moment—a separation of positive and negative charges, making it a polar molecule. This polarity influences its solubility in water (a polar solvent) and its interaction with other molecules.
Properties of Ammonia: A Detailed Overview
Ammonia exhibits several key properties that are crucial for its various applications:
Physical Properties:
-
Colorless Gas: Under standard conditions, ammonia is a colorless gas.
-
Pungent Odor: It has a very strong, characteristic pungent odor.
-
Solubility in Water: Ammonia is highly soluble in water, forming ammonium hydroxide (NH₄OH), a weak base.
-
Boiling Point: Ammonia has a relatively low boiling point (-33.34 °C), meaning it readily transitions from a liquid to a gas at relatively low temperatures.
-
Density: It is less dense than air.
Chemical Properties:
-
Weak Base: Ammonia acts as a weak base, accepting protons (H⁺) from acids. This property is fundamental to its use in many industrial and household applications.
-
Reactivity: It readily reacts with many acids to form ammonium salts.
-
Reducing Agent: Ammonia can act as a reducing agent, donating electrons to other substances.
-
Formation of Complexes: It can form coordination complexes with metal ions.
-
Combustion: Ammonia burns in oxygen to produce nitrogen oxides and water.
Wide-ranging Applications of Ammonia
Ammonia’s unique properties have led to its widespread use across various industries:
Industrial Applications:
-
Fertilizer Production: This is by far the largest application of ammonia. It serves as a crucial component in the production of nitrogen-based fertilizers, which are essential for boosting agricultural yields globally. Ammonia is converted into various nitrogen-containing fertilizers like urea, ammonium nitrate, and ammonium phosphate.
-
Refrigeration: Ammonia's low boiling point makes it an effective refrigerant in industrial refrigeration systems.
-
Cleaning Agent: Ammonia is used as a cleaning agent, especially in household products, due to its ability to dissolve grease and grime.
-
Production of other Chemicals: It serves as a building block for the synthesis of numerous other chemicals, including nitric acid, hydrazine, and various amines.
-
Textile Industry: Ammonia is used in the treatment of textiles, improving their quality and properties.
Other Uses:
-
Water Treatment: Ammonia is sometimes used in water treatment to regulate the pH level and remove chlorine.
-
Medical Applications: Ammonia is used in some medical applications, primarily as a respiratory stimulant.
-
Laboratory Reagent: It's a common laboratory reagent used in various chemical reactions and analyses.
Safety Precautions When Handling Ammonia
Ammonia, while crucial for various applications, can pose health hazards if handled improperly. Exposure to high concentrations of ammonia gas can lead to respiratory irritation, coughing, and even severe lung damage. Therefore, it's crucial to adhere to strict safety protocols:
-
Ventilation: Ensure adequate ventilation in areas where ammonia is used or stored.
-
Personal Protective Equipment (PPE): Wear appropriate PPE, including gloves, eye protection, and respirators, to minimize the risk of exposure.
-
Storage: Store ammonia in well-ventilated areas away from sources of ignition.
-
Emergency Procedures: Develop and practice emergency procedures in case of ammonia leaks or spills.
Conclusion: Ammonia - A Crucial Compound in Modern Society
In conclusion, ammonia is unequivocally a compound, not an element or a mixture. Its fixed composition, strong chemical bonds, and distinct properties firmly establish its classification as a compound. The unique properties of ammonia, along with its availability and relatively low cost, have fueled its widespread applications in various industries, notably fertilizer production, refrigeration, and cleaning. While its usefulness is undeniable, it's vital to remember the importance of safety precautions when handling ammonia due to its potential health hazards. Understanding the chemical nature of ammonia, its properties, and its appropriate handling procedures is crucial for its safe and effective utilization.
Latest Posts
Latest Posts
-
People Who Buy Stock In A Company Are Known As
Mar 15, 2025
-
The Cell Walls Of Fungi Are Made Up Of Cellulose
Mar 15, 2025
-
What Is The Basic Unit Of Heredity
Mar 15, 2025
-
9 Is What Percent Of 72
Mar 15, 2025
-
How Many Valence Electrons Are In H
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Is Ammonia An Element Compound Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
