Is Air An Element Compound Or Mixture
News Leon
Mar 18, 2025 · 6 min read
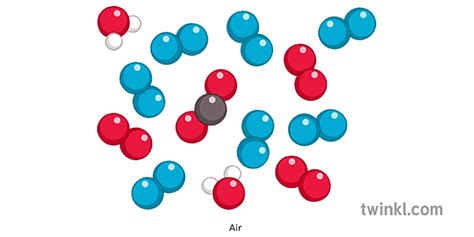
Table of Contents
Is Air an Element, Compound, or Mixture? A Deep Dive into Atmospheric Composition
The question, "Is air an element, compound, or mixture?" seems deceptively simple. We breathe it every day, yet the true nature of air is far more complex than many realize. The answer, unequivocally, is that air is a mixture. But understanding why requires exploring the fundamental differences between elements, compounds, and mixtures, and delving into the detailed composition of the Earth's atmosphere.
Understanding the Fundamentals: Elements, Compounds, and Mixtures
Before diving into the specifics of air, let's establish a clear understanding of the three classifications of matter:
Elements: The Building Blocks of Matter
Elements are the purest forms of matter. They are substances that cannot be broken down into simpler substances by chemical means. Each element is defined by the number of protons in its atomic nucleus, its atomic number. Examples of elements include oxygen (O), nitrogen (N), hydrogen (H), and iron (Fe). They exist as individual atoms or in specific arrangements (like diatomic molecules such as O₂ or N₂).
Compounds: Elements Bound Together
Compounds are formed when two or more different elements chemically combine in fixed proportions. These elements are bonded together through chemical bonds, creating a new substance with unique properties different from the individual elements. For example, water (H₂O) is a compound formed from the chemical combination of hydrogen and oxygen. The properties of water are vastly different from those of hydrogen gas and oxygen gas. The ratio of hydrogen to oxygen is always 2:1 in a water molecule. This fixed ratio is a defining characteristic of compounds.
Mixtures: A Blend of Substances
Mixtures are physical combinations of two or more substances that are not chemically bonded. The components of a mixture retain their individual properties and can be separated by physical methods, such as filtration, distillation, or evaporation. Unlike compounds, mixtures don't have a fixed composition; the ratio of components can vary. For example, a mixture of salt and sand can have varying amounts of each substance. Air, as we will see, falls squarely into this category.
The Composition of Air: A Complex Mixture
Air is a complex mixture of various gases, along with tiny particles of solids and liquids. Its composition is not uniform and varies depending on location, altitude, and weather conditions. However, we can describe its typical composition at sea level:
Major Components of Air:
-
Nitrogen (N₂): This makes up approximately 78% of the Earth's atmosphere. Nitrogen is relatively inert, meaning it doesn't readily react with other substances. Its role in the atmosphere is primarily to dilute the highly reactive oxygen.
-
Oxygen (O₂): Oxygen constitutes about 21% of air. It is crucial for respiration in most living organisms and essential for combustion. Oxygen is a highly reactive element and plays a critical role in many chemical processes.
-
Argon (Ar): This inert noble gas makes up around 0.93% of the atmosphere. It is relatively unreactive and plays a minor role in atmospheric processes.
Minor Components of Air:
Several other gases are present in smaller concentrations:
-
Carbon Dioxide (CO₂): Though only present in around 0.04%, carbon dioxide is a vital greenhouse gas that plays a crucial role in regulating Earth's temperature. Its concentration is increasing due to human activities, leading to concerns about climate change.
-
Neon (Ne), Helium (He), Methane (CH₄), Krypton (Kr), Hydrogen (H₂), Xenon (Xe), Ozone (O₃): These gases are present in trace amounts, but they still play important roles in atmospheric processes. For example, ozone in the stratosphere protects us from harmful ultraviolet radiation. Methane, a potent greenhouse gas, is released by various natural and human activities.
Variable Components of Air:
The following components vary significantly depending on location and time:
-
Water Vapor (H₂O): The amount of water vapor in the air varies greatly depending on temperature and humidity. Warm, moist air can contain significantly more water vapor than cold, dry air.
-
Aerosols: These are tiny solid or liquid particles suspended in the air, including dust, pollen, sea salt, and pollutants. Aerosols can affect air quality, cloud formation, and even climate.
Why Air is a Mixture, Not a Compound or Element
Air definitively qualifies as a mixture for several key reasons:
-
Variable Composition: The proportions of the gases in air are not fixed. The amount of water vapor, for instance, fluctuates considerably. This variability is a hallmark of mixtures, not compounds.
-
Retention of Individual Properties: The gases in air retain their individual chemical properties. Oxygen still supports combustion, nitrogen remains relatively inert, and argon remains a noble gas, even when mixed together in the atmosphere. In a compound, the constituent elements lose their individual characteristics and form a new substance with entirely different properties.
-
Physical Separation: The components of air can be separated using physical methods. For example, fractional distillation can be used to separate the different gases based on their boiling points. This is not possible with compounds, which require chemical reactions to be broken down into their constituent elements.
-
No Chemical Bonds: The gases in air are not chemically bonded to each other. They exist independently and simply occupy the same space. In a compound, atoms are linked together by strong chemical bonds, forming a stable molecule with distinct properties.
The Importance of Understanding Air's Composition
Understanding the composition of air is crucial for several reasons:
-
Environmental Monitoring: Tracking the concentrations of gases like carbon dioxide, methane, and ozone is essential for monitoring air quality and understanding the impacts of human activities on the environment.
-
Climate Change Research: Understanding the role of greenhouse gases in regulating Earth's temperature is crucial for addressing the challenge of climate change.
-
Respiratory Health: The composition of air directly impacts human health. Pollutants in the air can cause respiratory problems and other health issues.
-
Technological Applications: The properties of the gases in air are exploited in various technologies, from industrial processes to medical equipment.
Conclusion: Air – A Dynamic and Vital Mixture
In conclusion, air is unequivocally a mixture, not a compound or an element. Its complex composition, variable nature, and the ability to separate its components physically all point to this classification. Understanding this fundamental aspect of our environment is crucial for a wide range of scientific, technological, and environmental applications, emphasizing the vital role air plays in sustaining life on Earth. Further research and monitoring of its composition are essential for safeguarding the health of our planet and its inhabitants. The dynamic and ever-changing nature of this crucial mixture ensures its continued study and relevance for years to come. The seemingly simple question of whether air is an element, compound, or mixture opens the door to a fascinating and complex world of atmospheric science and chemistry.
Latest Posts
Latest Posts
-
Why Is Boiling Water A Physical Change
Mar 19, 2025
-
What Is The Product Of The Following Sequence Of Reactions
Mar 19, 2025
-
How Are Weathering And Erosion Difference
Mar 19, 2025
-
Double Replacement Examples In Real Life
Mar 19, 2025
-
The Study Of Population Is Called
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is Air An Element Compound Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
