Why Is Boiling Water A Physical Change
News Leon
Mar 19, 2025 · 5 min read
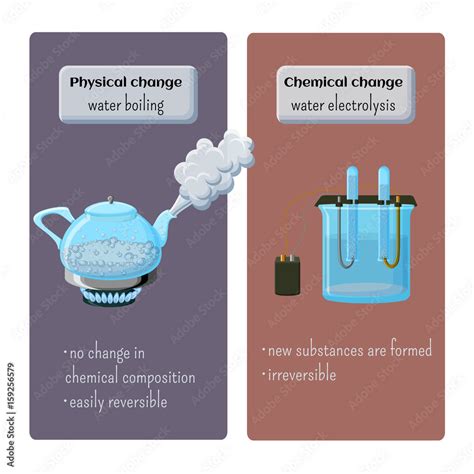
Table of Contents
Why is Boiling Water a Physical Change? Understanding the Science Behind It
Boiling water is a classic example of a physical change, not a chemical change. This seemingly simple process holds profound implications in understanding the fundamental principles of matter and its transformations. This article will delve deep into the science behind why boiling water is categorized as a physical change, exploring the concepts of phase transitions, molecular structure, and the reversibility of the process. We'll also examine common misconceptions and address frequently asked questions to solidify your understanding.
Understanding Physical vs. Chemical Changes
Before diving into the specifics of boiling water, let's establish a clear distinction between physical and chemical changes. This fundamental understanding is crucial for grasping the nature of the boiling process.
Physical Changes: These changes affect the form or appearance of a substance but do not alter its chemical composition. The molecules themselves remain the same; only their arrangement or state of matter changes. Examples include melting ice, dissolving sugar in water, or bending a metal wire. These changes are often reversible.
Chemical Changes: These changes involve a rearrangement of atoms and molecules, resulting in the formation of new substances with different chemical properties. Chemical changes are often irreversible and are frequently accompanied by observable changes like heat release or gas production. Examples include burning wood, rusting iron, or cooking an egg.
The Process of Boiling Water: A Detailed Look
Boiling water is a phase transition, specifically from the liquid phase to the gaseous phase, also known as vaporization. This process occurs when the water molecules gain enough kinetic energy to overcome the intermolecular forces holding them together in the liquid state.
Molecular Behavior in Liquid Water
In liquid water, water molecules (H₂O) are constantly moving and interacting. They are held together by relatively weak intermolecular forces, primarily hydrogen bonds. These bonds are responsible for water's unique properties, such as its high boiling point and surface tension. The molecules are close together, but they are not fixed in a rigid structure, allowing for fluidity.
The Role of Heat Energy
When heat is applied to water, the molecules absorb energy, increasing their kinetic energy – essentially making them move faster. This increased kinetic energy overcomes the attractive forces between the molecules. As the temperature rises, more and more molecules gain sufficient energy to escape the liquid phase and transition into the gaseous phase (water vapor or steam).
Reaching the Boiling Point
The boiling point of water at standard atmospheric pressure (1 atm) is 100°C (212°F). At this temperature, the vapor pressure of water (the pressure exerted by the water vapor molecules) equals the atmospheric pressure. This allows the water molecules to overcome the atmospheric pressure and escape as steam, forming bubbles within the liquid. Before reaching the boiling point, the water heats up, but evaporation occurs only at the surface.
Boiling: A Phase Transition, Not a Decomposition
Crucially, the water molecules themselves remain H₂O throughout the boiling process. There is no change in their chemical composition. They simply transition from a more closely packed liquid state to a more dispersed gaseous state. This is why boiling water is considered a physical change. No new chemical substances are formed. The same molecules that were in liquid water are now in gaseous water (steam).
Reversibility: A Key Indicator of a Physical Change
One of the hallmarks of a physical change is its reversibility. In the case of boiling water, this is demonstrated through condensation. As water vapor cools, the water molecules lose kinetic energy, and the intermolecular forces become strong enough to draw the molecules back together, forming liquid water. This process is the exact reverse of boiling. You can easily collect the steam, allow it to cool, and recover the liquid water—clear evidence of a reversible physical change.
Addressing Common Misconceptions
Some misunderstandings frequently arise regarding boiling water and its classification as a physical change. Let's address a few of them:
Misconception 1: The formation of steam is a chemical reaction.
Clarification: The transformation of liquid water to steam involves only a change in state, not a chemical reaction. The chemical formula remains H₂O.
Misconception 2: Boiling water changes its properties drastically, therefore it's a chemical change.
Clarification: While the physical properties like density and volume change during boiling, the chemical properties, such as flammability or reactivity, remain the same. The substance is still water; its fundamental nature hasn't been altered.
Misconception 3: Bubbles are indicative of a chemical reaction.
Clarification: The bubbles observed during boiling are simply water vapor escaping the liquid phase. They do not represent the formation of new chemical substances.
Beyond Boiling: Other Physical Changes of Water
Water undergoes several other physical changes, all demonstrating its reversibility and lack of change in chemical composition:
- Melting: The transition from solid ice to liquid water.
- Freezing: The transition from liquid water to solid ice.
- Sublimation: The transition directly from solid ice to gaseous water vapor (without passing through the liquid phase).
- Deposition: The transition directly from gaseous water vapor to solid ice.
These are all physical changes because they only involve alterations in the physical state of water, not its chemical identity.
Further Applications and Implications
The understanding that boiling water is a physical change is essential in various fields:
- Chemistry: It provides a fundamental illustration of phase transitions and the behavior of molecules under different conditions.
- Cooking: The boiling of water is crucial in numerous culinary applications, from cooking pasta to brewing tea. Understanding the process helps control the temperature and achieve desired results.
- Engineering: Phase transitions like boiling are considered in various engineering applications, including power generation (steam turbines), refrigeration, and chemical processing.
- Meteorology: The phase transitions of water are fundamental to weather patterns, including cloud formation, precipitation, and evaporation.
Conclusion: The Simplicity and Significance of Boiling Water
The seemingly simple process of boiling water serves as a powerful illustration of a physical change. It highlights the distinction between changes in physical state and changes in chemical composition. The reversibility of the process, the absence of new substance formation, and the retention of the original chemical identity of water unequivocally classify boiling as a physical change. This understanding forms a cornerstone of scientific knowledge and has wide-ranging applications across diverse fields. Through careful observation and the application of scientific principles, we can easily and convincingly demonstrate why boiling water remains, fundamentally, a physical phenomenon.
Latest Posts
Latest Posts
-
In A Two Digit Number The Tens Digit
Mar 19, 2025
-
In The Figure Pq Is Parallel To Rs
Mar 19, 2025
-
I Am Who I Am Essay
Mar 19, 2025
-
Inertia Is A Property Of Matter
Mar 19, 2025
-
Which Chambers Of The Heart Have Thicker Walls
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Why Is Boiling Water A Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
