Ions That Carry A Positive Charge Are Called
News Leon
Apr 06, 2025 · 6 min read
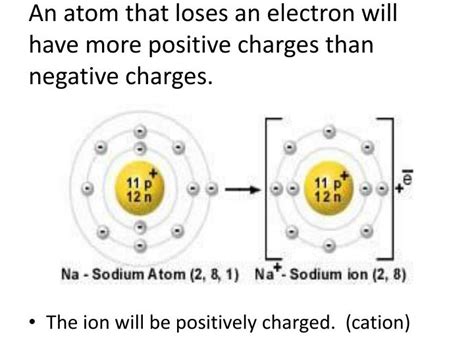
Table of Contents
Ions That Carry a Positive Charge Are Called Cations: A Deep Dive into their Properties and Significance
Ions, the electrically charged atoms or molecules, form the backbone of countless chemical processes across various disciplines, from biology to materials science. Understanding the nature of ions, particularly those carrying a positive charge, is crucial to grasping the intricacies of chemical reactions, electrical conductivity, and many other phenomena. This article delves into the world of cations, ions that carry a positive charge, exploring their formation, properties, and extensive roles in diverse fields.
What are Cations?
Cations are atomic or molecular species that carry a net positive electrical charge. This positive charge arises when an atom or molecule loses one or more electrons. The loss of negatively charged electrons results in an imbalance, leaving behind more protons (positively charged particles in the nucleus) than electrons, hence the positive charge. The number of positive charges indicates the cation's valency or oxidation state. For instance, a sodium ion (Na⁺) has a +1 charge, indicating it has lost one electron, while a calcium ion (Ca²⁺) has a +2 charge, signifying the loss of two electrons.
Formation of Cations
Cation formation predominantly occurs through ionization, a process where an atom or molecule loses one or more valence electrons. This process usually involves the transfer of electrons to a more electronegative atom or molecule, often a nonmetal. The driving force behind cation formation is the tendency of atoms to achieve a stable electron configuration, often resembling that of a noble gas (group 18 elements in the periodic table). Atoms with a few valence electrons readily lose these electrons to achieve a stable octet (eight electrons in the outermost shell) or duplet (two electrons for very small atoms like hydrogen and helium).
Examples of Cation Formation:
-
Sodium (Na) to Sodium Ion (Na⁺): Sodium, an alkali metal with one valence electron, readily loses this electron to become a sodium ion (Na⁺), achieving the stable electron configuration of neon.
-
Magnesium (Mg) to Magnesium Ion (Mg²⁺): Magnesium, an alkaline earth metal with two valence electrons, loses both electrons to form the magnesium ion (Mg²⁺), attaining the stable electron configuration of neon.
-
Aluminum (Al) to Aluminum Ion (Al³⁺): Aluminum, a group 13 element, loses three valence electrons to form the aluminum ion (Al³⁺), achieving the stable electron configuration of neon.
-
Transition Metal Cations: Transition metals can form cations with varying charges. For example, iron can form Fe²⁺ and Fe³⁺ ions, depending on the conditions. This variable valency is a characteristic feature of transition metals.
Properties of Cations
The properties of cations are significantly influenced by their charge and size.
Charge Density:
The charge density of a cation, the ratio of its charge to its size, plays a crucial role in determining its reactivity and interactions with other species. Smaller cations with higher charges have higher charge densities, making them more strongly polarizing and influencing their interactions with anions (negatively charged ions) and other molecules.
Size:
The size of a cation is inversely proportional to its charge. As more electrons are lost, the remaining electrons are pulled closer to the nucleus by the increased positive charge, resulting in a smaller ionic radius. This decrease in size influences its reactivity and its ability to fit into specific crystal lattices or biological structures.
Polarizing Power:
Cations with high charge density have a strong polarizing power, meaning they can distort the electron cloud of an anion or a polar molecule. This polarization influences the chemical bonding and the overall properties of the resulting compounds. For example, the high polarizing power of small, highly charged cations can lead to covalent character in ionic compounds.
Hydration:
In aqueous solutions, cations become hydrated, meaning they are surrounded by water molecules. The water molecules are oriented with their negative dipole ends towards the positive charge of the cation. The extent of hydration depends on the charge density of the cation; smaller, highly charged cations have stronger hydration spheres. This hydration affects the cation's mobility and reactivity in solution.
Significance of Cations in Various Fields
Cations play essential roles across various scientific and technological disciplines.
Biology:
Cations are vital components of biological systems. Sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and magnesium (Mg²⁺) ions are essential for numerous physiological processes, including nerve impulse transmission, muscle contraction, enzyme activity, and maintaining osmotic balance. Their precise concentrations within and outside cells are tightly regulated to ensure proper functioning of the organism. Imbalances in cation levels can lead to various health problems.
Chemistry:
Cations are essential participants in numerous chemical reactions. Their involvement in redox (reduction-oxidation) reactions is crucial in energy production and storage processes. The formation of ionic compounds, crucial in materials science, depends entirely on the electrostatic attraction between cations and anions. The properties of ionic compounds, such as melting point, solubility, and conductivity, are significantly influenced by the properties of the constituent cations and anions.
Materials Science:
Cations are fundamental building blocks in various materials. The arrangement of cations and anions in crystal structures determines the properties of many solids, such as ceramics, metals, and alloys. The electronic and optical properties of these materials are also significantly influenced by the cations present. For instance, the color of gemstones often arises from the presence of specific transition metal cations in the crystal lattice.
Environmental Science:
Cation analysis is crucial in environmental monitoring. The presence and concentrations of various cations in water bodies, soil, and air provide valuable information about environmental pollution and its impact on ecosystems. Understanding cation transport and cycling in the environment is critical for managing environmental resources and mitigating pollution.
Examples of Specific Cations and their Roles:
-
Sodium (Na⁺): Crucial for nerve impulse transmission, fluid balance, and muscle function. Used extensively in sodium-ion batteries as an alternative to lithium-ion batteries.
-
Potassium (K⁺): Essential for nerve impulse transmission, muscle contraction, and maintaining cell membrane potential. Plays a critical role in plant growth and nutrient uptake.
-
Calcium (Ca²⁺): Important for bone structure, muscle contraction, blood clotting, and nerve transmission. Used in various industrial applications, including cement production and water treatment.
-
Magnesium (Mg²⁺): Essential cofactor for many enzymes, involved in muscle function, protein synthesis, and DNA replication. Used in alloys for increased strength and lightness.
-
Iron (Fe²⁺ and Fe³⁺): Crucial for oxygen transport in hemoglobin and myoglobin. Used extensively in steel production and various industrial catalysts.
-
Copper (Cu⁺ and Cu²⁺): Important in several enzyme systems, including those involved in respiration and antioxidant defense. Used in electrical wiring and various alloys.
Conclusion:
Cations, ions carrying a positive charge, are ubiquitous across various scientific and technological domains. Their formation, properties, and interactions determine the characteristics of numerous materials, chemical reactions, and biological processes. Understanding the behavior of cations is essential for advancements in numerous fields, from medicine and materials science to environmental science and energy technologies. Further research into the properties and applications of cations will undoubtedly lead to new discoveries and innovations in the future. The exploration of new cation-based materials and technologies is an exciting and rapidly evolving area of research with significant implications for the future. The world of cations is vast and multifaceted, constantly presenting new challenges and opportunities for scientific exploration.
Latest Posts
Latest Posts
-
A Quadrilateral In Which The Diagonals Bisect Each Other
Apr 07, 2025
-
Units For Rate Constant K Third Order
Apr 07, 2025
-
Which Reaction Is An Example Of Heterogeneous Catalysis
Apr 07, 2025
-
What Is The Role Of The Brain In Reflex Action
Apr 07, 2025
-
Which Of The Following Is Equal To 1
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Ions That Carry A Positive Charge Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
