Increasing The Temperature Increases The Rate Of A Reaction By
News Leon
Apr 07, 2025 · 5 min read
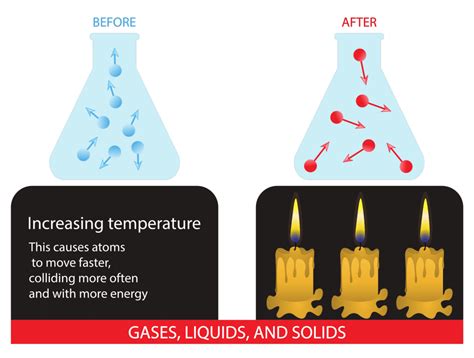
Table of Contents
Increasing the Temperature Increases the Rate of a Reaction: A Deep Dive into Collision Theory and Activation Energy
The speed at which a chemical reaction proceeds, known as its reaction rate, is influenced by a multitude of factors. Among these, temperature stands out as a particularly significant variable. A fundamental principle in chemistry dictates that increasing the temperature increases the rate of a reaction. But why is this the case? This article delves into the underlying mechanisms, exploring the concepts of collision theory, activation energy, and the Arrhenius equation, to provide a comprehensive understanding of this crucial relationship.
Collision Theory: The Foundation of Reaction Rates
At the heart of understanding how temperature affects reaction rates lies collision theory. This theory posits that for a reaction to occur, reactant particles must collide with each other. However, not all collisions are created equal. A successful collision, one that leads to the formation of products, must meet two crucial criteria:
1. Sufficient Energy: Overcoming the Activation Energy Barrier
Reactant particles possess a certain amount of kinetic energy. This energy must exceed a minimum threshold known as the activation energy (Ea). The activation energy represents the energy barrier that reactant molecules must overcome to transform into products. Think of it as the "hill" that reactants must climb before they can roll down to the product valley. If the colliding particles possess insufficient kinetic energy, they will simply bounce off each other without reacting.
2. Proper Orientation: Achieving Effective Collisions
Even if colliding particles possess sufficient kinetic energy, their orientation during the collision matters. The particles must collide in a specific orientation that allows the necessary bonds to break and new bonds to form. An ineffective collision, even with sufficient energy, will result in the reactants simply rebounding without reaction.
Temperature's Role in Enhancing Reaction Rates
Temperature is directly linked to the kinetic energy of particles. Higher temperatures mean higher average kinetic energies. This increased kinetic energy has two significant consequences for reaction rates:
1. Increased Frequency of Collisions
With higher kinetic energies, particles move faster and more frequently. This leads to a greater number of collisions per unit time, increasing the overall probability of successful collisions.
2. Increased Proportion of Effective Collisions
The increased average kinetic energy also means a larger proportion of collisions will possess sufficient energy to overcome the activation energy barrier (Ea). More collisions will have the energy needed to initiate the reaction. This is the most significant factor contributing to the accelerated reaction rate at higher temperatures.
Visualizing the Effect: Boltzmann Distribution
The Boltzmann distribution provides a visual representation of the relationship between temperature and the distribution of kinetic energies among particles. At lower temperatures, the curve is narrower and peaks at a lower kinetic energy. A smaller fraction of particles possesses sufficient energy to overcome the activation energy. As the temperature increases, the curve broadens and shifts to higher energies. A significantly larger fraction of particles now possesses the necessary kinetic energy to react, leading to a dramatic increase in the reaction rate.
The Arrhenius Equation: A Quantitative Relationship
The relationship between temperature and reaction rate is mathematically described by the Arrhenius equation:
k = A * e^(-Ea/RT)
Where:
- k is the rate constant (a measure of the reaction rate)
- A is the pre-exponential factor (related to the frequency of collisions and the orientation factor)
- Ea is the activation energy
- R is the ideal gas constant
- T is the temperature in Kelvin
This equation shows that the rate constant (and therefore the reaction rate) increases exponentially with temperature. A small increase in temperature can lead to a substantial increase in the reaction rate, especially for reactions with high activation energies.
Examples Illustrating the Temperature Dependence of Reaction Rates
Numerous everyday examples demonstrate the profound influence of temperature on reaction rates:
- Cooking: Food cooks faster at higher temperatures because the rate of chemical reactions involved in the cooking process (like protein denaturation) increases significantly.
- Spoilage of Food: Food spoils more quickly at higher temperatures due to the accelerated rates of microbial growth and enzymatic reactions.
- Combustion: Fire, a combustion reaction, requires a sufficient activation energy. The presence of a flame provides the necessary initial energy, and increased temperature dramatically accelerates the reaction.
- Industrial Processes: Many industrial chemical processes are carried out at elevated temperatures to enhance reaction rates and improve efficiency.
Beyond the Basics: Catalysis and Other Factors
While temperature is a crucial factor, other elements also influence reaction rates. Catalysis, for example, lowers the activation energy, thus accelerating the reaction without altering the temperature. Catalysts provide an alternative reaction pathway with a lower energy barrier, making it easier for reactants to transform into products. The concentration of reactants, the presence of inhibitors, and the surface area (in heterogeneous reactions) also play important roles.
Conclusion: Temperature – A Key Regulator of Chemical Reactions
In summary, increasing the temperature increases the rate of a reaction because it leads to both a higher frequency of collisions and a greater proportion of collisions with sufficient energy to overcome the activation energy barrier. This relationship is fundamental to chemistry and has far-reaching implications in various fields, from cooking and food preservation to industrial processes and environmental science. Understanding the principles of collision theory, activation energy, and the Arrhenius equation provides a robust framework for comprehending and predicting the effects of temperature on chemical reaction rates. Further research into the intricacies of these principles continues to refine our understanding of chemical kinetics and its crucial role in the world around us. The impact of temperature on reaction rates is a cornerstone concept in chemistry, illustrating the dynamic and interconnected nature of molecular interactions. This comprehensive understanding is vital for advancements in various scientific disciplines and technological applications. The ongoing exploration of this fundamental principle promises further insights into the fascinating realm of chemical reactions and their dependence on environmental conditions.
Latest Posts
Latest Posts
-
Replication Is Called Semi Conservative Because
Apr 08, 2025
-
Oxidation Number Of C In C2o4 2
Apr 08, 2025
-
Examples Of Right Angles In Real Life
Apr 08, 2025
-
How Many Radians Is 60 Degrees
Apr 08, 2025
-
Do Cephalopods Have A Closed Circulatory System
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Increasing The Temperature Increases The Rate Of A Reaction By . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.