In Which Of The Following Processes Will Be Negative
News Leon
Mar 17, 2025 · 6 min read
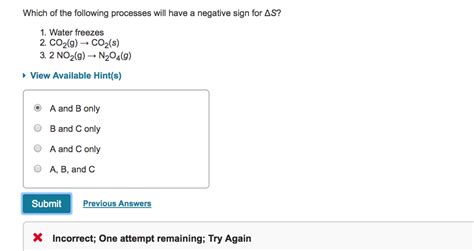
Table of Contents
In Which of the Following Processes Will ΔS be Negative? Understanding Entropy Changes
Entropy (ΔS), a crucial concept in thermodynamics and chemistry, measures the randomness or disorder of a system. A positive ΔS indicates an increase in disorder, while a negative ΔS signifies a decrease in disorder, meaning the system becomes more ordered. Predicting the sign of ΔS for a given process requires careful consideration of the changes in molecular arrangements and energy dispersal. This article will delve into various processes, explaining why some exhibit negative entropy changes.
Understanding Entropy: A Quick Recap
Before diving into specific examples, let's briefly revisit the fundamental principles of entropy. Entropy is a state function, meaning its value depends only on the initial and final states of the system, not the path taken. The second law of thermodynamics states that the total entropy of an isolated system can only increase over time or remain constant in ideal cases where the system is in a steady state or undergoing a reversible process. In simpler terms, systems naturally tend towards greater disorder.
Processes with Negative ΔS: A Detailed Analysis
Several types of processes typically lead to a decrease in entropy (negative ΔS). These include:
1. Phase Transitions from Gas to Liquid or Solid
The transition from a gas to a liquid (condensation) or from a liquid to a solid (freezing) always results in a negative change in entropy. This is because gases possess significantly higher entropy than liquids or solids due to their greater molecular freedom and randomness of motion. In the gaseous state, molecules are widely dispersed and move independently. When a gas condenses or freezes, molecules become more ordered, confined to a smaller volume, and restricted in their movement, hence the decrease in entropy.
Example: The condensation of water vapor into liquid water (H₂O(g) → H₂O(l)) has a negative ΔS. The molecules in gaseous water are highly disordered and move freely, while in liquid water, they are more closely packed and exhibit restricted motion.
2. Chemical Reactions Leading to Fewer Molecules
Chemical reactions where the number of gaseous molecules decreases often exhibit negative ΔS. This stems from the loss of translational freedom and randomness associated with fewer gas particles.
Example: The reaction between two moles of hydrogen gas and one mole of oxygen gas to form two moles of water vapor: 2H₂(g) + O₂(g) → 2H₂O(g)
While this reaction involves gases throughout, the number of gas molecules decreases from 3 to 2. This reduction in the number of independent moving particles leads to a negative entropy change. However, it's crucial to note that the magnitude of the entropy change also depends on the specific temperature and pressure conditions.
Example: The Haber-Bosch process for ammonia synthesis (N₂(g) + 3H₂(g) → 2NH₃(g)) also shows a negative ΔS. Four moles of reactant gases combine to yield two moles of product gas, resulting in a decrease in the total number of gas molecules and thus, a more ordered system.
3. Dissolution of Gases in Liquids
When a gas dissolves in a liquid, its molecules become more ordered and less randomly dispersed. This is because the gas molecules are now confined within the solvent's structure, losing their translational freedom. Consequently, the dissolution of gases in liquids typically results in a negative ΔS.
Example: The dissolution of carbon dioxide (CO₂) in water (CO₂(g) → CO₂(aq)) is an example. The gaseous CO₂ molecules, initially highly disordered, are now solvated and their movement is more restricted, leading to a decrease in entropy.
4. Precipitation Reactions
Precipitation reactions, where soluble ionic compounds form an insoluble solid precipitate, are characterized by a decrease in entropy. The dissolved ions, initially randomly dispersed in the solution, become organized into a structured solid lattice. This highly ordered arrangement significantly reduces the system's randomness.
Example: The reaction between silver nitrate (AgNO₃) and sodium chloride (NaCl) to form silver chloride precipitate (AgCl) and sodium nitrate (NaNO₃) : AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
In this example, the dissolved ions become incorporated into the more ordered solid AgCl lattice.
5. Formation of Complex Ions
The formation of complex ions from simpler ions also typically leads to a negative ΔS. This is because the independent ions are now linked together in a specific arrangement within the complex, reducing the system's overall randomness.
Example: The formation of a hexaaquacopper(II) complex ion [Cu(H₂O)₆]²⁺ from Cu²⁺ ions and water molecules. The free Cu²⁺ and H₂O molecules have greater randomness compared to the structured complex ion.
6. Reactions Leading to a Decrease in Degrees of Freedom
Processes that restrict the movement or vibrational freedom of molecules tend to exhibit negative ΔS. This can involve the formation of strong intermolecular bonds or a decrease in the number of accessible energy levels.
Factors Affecting the Magnitude of Negative ΔS
Several factors influence the magnitude of the negative entropy change:
-
Temperature: Lower temperatures generally lead to larger negative entropy changes because molecules have less kinetic energy and are more easily constrained into ordered arrangements.
-
Pressure: Increased pressure can favor processes with negative ΔS because it forces molecules closer together, reducing their freedom of movement.
-
Nature of the species involved: The specific chemical nature of the substances involved—their size, shape, and intermolecular forces—influences the extent of ordering and hence the magnitude of the entropy change.
Distinguishing ΔS from ΔH and ΔG
It's crucial to differentiate entropy (ΔS) from enthalpy (ΔH) and Gibbs free energy (ΔG). Enthalpy represents the heat content of a system, while Gibbs free energy combines enthalpy and entropy to predict the spontaneity of a process. The relationship between these three thermodynamic quantities is expressed by the equation:
ΔG = ΔH - TΔS
where T is the absolute temperature. A negative ΔG indicates a spontaneous process, while a positive ΔG signifies a non-spontaneous process. The sign of ΔS contributes to the overall spontaneity, particularly at higher temperatures.
Conclusion: Predicting Negative Entropy Changes
Predicting the sign of ΔS for a particular process requires analyzing the overall changes in the system's order and disorder. Processes that lead to decreased randomness, such as phase transitions from gas to liquid or solid, formation of precipitates, and reactions producing fewer gas molecules, are likely to exhibit negative entropy changes. While entropy change is a vital factor in determining reaction spontaneity, it needs to be considered in conjunction with enthalpy changes and temperature to fully understand the thermodynamics of a process. By carefully considering the factors discussed above, you can effectively predict whether a process will lead to a decrease in entropy (negative ΔS). Remember that the quantitative value of ΔS can be calculated using appropriate thermodynamic data, providing a more precise understanding of entropy changes in specific reactions and processes.
Latest Posts
Latest Posts
-
Which Statement About Natural Selection Is True
Mar 18, 2025
-
Which Chamber Of Heart Has Thickest Wall
Mar 18, 2025
-
How Many Feet Is 1 2 Miles
Mar 18, 2025
-
How Many Valence Electrons Does Mn Have
Mar 18, 2025
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about In Which Of The Following Processes Will Be Negative . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
