How To Find The Moles Of An Element
News Leon
Apr 01, 2025 · 6 min read
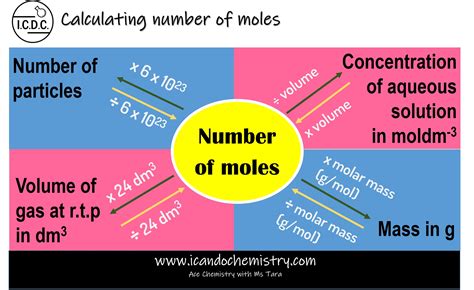
Table of Contents
How to Find the Moles of an Element: A Comprehensive Guide
Determining the number of moles of an element is a fundamental concept in chemistry, crucial for various calculations and experiments. Understanding this process is essential for anyone working with chemical reactions and stoichiometry. This comprehensive guide will walk you through different methods and scenarios, ensuring you master this vital skill.
Understanding Moles and Avogadro's Number
Before diving into the calculations, let's establish a clear understanding of what a mole represents. A mole (mol) is a unit of measurement in chemistry that represents a specific number of particles, whether atoms, molecules, ions, or formula units. This number is known as Avogadro's number, approximately 6.022 x 10²³ particles per mole. Think of it like a dozen (12) but on a vastly larger scale. One mole of carbon atoms contains 6.022 x 10²³ carbon atoms, just as one dozen eggs contains 12 eggs.
Methods for Calculating Moles
The method used to determine the number of moles depends on the information available. Here are the most common approaches:
1. Using Mass and Molar Mass
This is arguably the most frequently used method. The molar mass (M) of an element is the mass of one mole of that element, expressed in grams per mole (g/mol). It's numerically equal to the atomic weight of the element found on the periodic table. For instance, the molar mass of carbon (C) is approximately 12.01 g/mol.
The formula for calculating moles (n) from mass (m) and molar mass (M) is:
n = m / M
Example: How many moles are there in 24.02 grams of carbon?
- m = 24.02 g
- M = 12.01 g/mol
- n = 24.02 g / 12.01 g/mol = 2 moles
Therefore, there are 2 moles of carbon in 24.02 grams.
This method is straightforward and widely applicable, making it a cornerstone of chemical calculations. Remember to always use the correct units (grams for mass and g/mol for molar mass) to ensure accurate results. Incorrect unit usage can lead to significant errors in your calculations.
2. Using the Number of Atoms or Molecules
If you know the actual number of atoms of an element, you can use Avogadro's number to calculate the number of moles. The formula is:
n = N / Nₐ
Where:
- n is the number of moles
- N is the number of atoms or molecules
- Nₐ is Avogadro's number (6.022 x 10²³)
Example: How many moles are present in 1.2044 x 10²⁴ atoms of oxygen?
- N = 1.2044 x 10²⁴ atoms
- Nₐ = 6.022 x 10²³ atoms/mol
- n = (1.2044 x 10²⁴ atoms) / (6.022 x 10²³ atoms/mol) = 2 moles
This method is particularly useful when dealing with microscopic quantities or when dealing with problems focused on the number of particles involved in a reaction.
3. Using Volume and Molar Volume (for Gases)
For gases at standard temperature and pressure (STP, 0°C and 1 atm), the molar volume is approximately 22.4 liters per mole (L/mol). This means that one mole of any ideal gas occupies 22.4 liters at STP. The formula is:
n = V / Vₘ
Where:
- n is the number of moles
- V is the volume of the gas in liters (L)
- Vₘ is the molar volume (22.4 L/mol at STP)
Important Note: This method is only applicable to gases at STP or conditions where the ideal gas law is reasonably accurate. Deviations from ideality become more significant at higher pressures and lower temperatures.
Example: What is the number of moles in 44.8 liters of nitrogen gas at STP?
- V = 44.8 L
- Vₘ = 22.4 L/mol
- n = 44.8 L / 22.4 L/mol = 2 moles
4. Using Concentration and Volume (for Solutions)
When dealing with solutions, the concentration (usually in molarity, M) and volume can be used to calculate the number of moles of solute present. Molarity is defined as moles of solute per liter of solution (mol/L). The formula is:
n = C x V
Where:
- n is the number of moles
- C is the concentration in molarity (mol/L)
- V is the volume of the solution in liters (L)
Example: How many moles of sodium chloride (NaCl) are present in 500 mL of a 0.5 M NaCl solution?
- First, convert the volume to liters: 500 mL = 0.5 L
- C = 0.5 mol/L
- V = 0.5 L
- n = 0.5 mol/L x 0.5 L = 0.25 moles
This method is crucial in various analytical and preparative chemistry techniques where precise control over the amount of solute is vital.
Addressing Common Challenges and Pitfalls
While the calculations themselves are relatively straightforward, certain challenges can arise:
- Unit Conversion: Always ensure all your units are consistent. Convert grams to kilograms, milliliters to liters, etc., as needed before plugging values into the formulas. Inconsistent units are a common source of error.
- Significant Figures: Pay close attention to significant figures throughout your calculations. Your final answer should reflect the precision of the measurements used.
- Ideal Gas Law Deviations: Remember that the molar volume (22.4 L/mol) is only accurate for ideal gases at STP. Significant deviations can occur under non-ideal conditions. In such cases, more complex equations, like the van der Waals equation, might be needed.
- Hydrates: If you're working with hydrates (compounds containing water molecules), remember to include the mass of the water molecules when calculating the molar mass. The molar mass will be higher due to the addition of water.
- Mixed Compounds: If dealing with a mixture of elements, carefully account for the individual molar masses and the relative proportions of each element in the mixture. You'll need additional information about the composition of the mixture to calculate the moles of individual elements.
Advanced Applications and Real-World Examples
The ability to calculate the number of moles is not just a theoretical exercise; it's fundamental to numerous real-world applications:
- Stoichiometry: This branch of chemistry relies heavily on mole calculations to determine the amounts of reactants and products in chemical reactions. It's crucial for balancing equations and predicting reaction yields.
- Titrations: In titrations, the number of moles of a substance is calculated to determine the concentration of an unknown solution.
- Gas Analysis: Mole calculations are vital in analyzing gas mixtures, such as those in atmospheric monitoring or industrial processes.
- Pharmaceutical Chemistry: Precise mole calculations are essential in pharmaceutical manufacturing and dosage control.
- Environmental Science: Calculating the moles of pollutants in environmental samples is critical for monitoring and controlling pollution.
Conclusion
Mastering the ability to determine the number of moles of an element is paramount for anyone pursuing a career in science or engineering, or for students studying chemistry. By understanding the different methods presented and paying close attention to detail regarding units and significant figures, you can confidently perform these vital calculations. Remember to carefully choose the appropriate formula based on the available information and consider potential challenges such as unit consistency and deviations from ideality. With practice, you will become proficient in using this fundamental concept to solve a wide array of chemical problems.
Latest Posts
Latest Posts
-
What Is 13 25 As A Decimal
Apr 02, 2025
-
How Many Months Are In Five Years
Apr 02, 2025
-
Geometric Mean Of 9 And 4
Apr 02, 2025
-
A Codon Consists Of How Many Bases
Apr 02, 2025
-
All Summer In A Day Ray Bradbury Summary
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Moles Of An Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
