How To Find Molarity From Absorbance
News Leon
Mar 23, 2025 · 5 min read
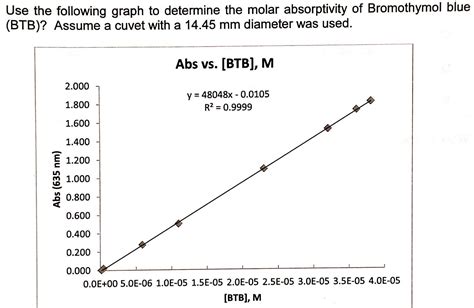
Table of Contents
How to Find Molarity from Absorbance: A Comprehensive Guide
Determining the concentration of a solution is a fundamental task in many scientific fields, from chemistry and biochemistry to environmental science and medicine. One powerful technique for this is spectrophotometry, which utilizes the relationship between a solution's absorbance of light and its concentration. This article provides a comprehensive guide on how to find molarity from absorbance, covering the underlying principles, practical steps, and potential challenges.
Understanding the Beer-Lambert Law: The Foundation of Spectrophotometry
The cornerstone of calculating molarity from absorbance is the Beer-Lambert Law, also known as Beer's Law. This law states that the absorbance of a solution is directly proportional to both the concentration of the analyte and the path length of the light through the solution. Mathematically, it's expressed as:
A = εbc
Where:
- A represents the absorbance of the solution (unitless). This is the measured value obtained from a spectrophotometer.
- ε is the molar absorptivity (or molar extinction coefficient) of the analyte at a specific wavelength. This is a constant that is specific to the substance being measured and the wavelength of light used. Its units are typically L mol⁻¹ cm⁻¹.
- b is the path length of the light through the solution, usually the width of the cuvette used in the spectrophotometer. This is typically 1 cm.
- c represents the concentration of the analyte in moles per liter (mol/L), which is the molarity we want to determine.
Practical Steps: Determining Molarity from Absorbance
Following these steps will guide you through the process:
1. Prepare Standard Solutions
Before measuring the absorbance of your unknown sample, you need to create a series of standard solutions with known concentrations of your analyte. This creates a calibration curve, which is essential for accurately determining the unknown concentration. The range of concentrations should bracket the expected concentration of your unknown sample.
Why a Calibration Curve? While the Beer-Lambert Law provides a theoretical relationship, real-world measurements often deviate slightly due to factors like instrumental limitations and solution imperfections. A calibration curve allows us to account for these deviations and improve accuracy.
2. Measure Absorbance of Standard Solutions
Using a spectrophotometer, measure the absorbance of each standard solution at a specific wavelength where your analyte shows strong absorbance. This wavelength is typically determined by finding the λmax (wavelength of maximum absorbance) for your analyte through a preliminary scan. Choosing λmax ensures maximum sensitivity and accuracy.
Important Considerations:
- Blank Solution: Always measure the absorbance of a blank solution (a solution containing everything except the analyte) first. This corrects for any absorbance from the solvent or other components. Subtract the blank's absorbance from all subsequent measurements.
- Cuvette: Use a clean, matched cuvette for each measurement to minimize errors.
- Temperature Control: Maintaining consistent temperature throughout the experiment is crucial for accurate results.
3. Create a Calibration Curve
Plot the absorbance values (y-axis) against the corresponding concentrations (x-axis) of your standard solutions. This should yield a linear relationship, ideally passing through the origin (0,0), confirming adherence to the Beer-Lambert Law.
Software and Analysis: You can use spreadsheet software like Microsoft Excel or Google Sheets to easily create the calibration curve and perform linear regression. The equation of the line will be in the form:
A = mC + b
Where:
- A is the absorbance
- m is the slope of the line (which is equivalent to εb)
- C is the concentration
- b is the y-intercept (ideally close to zero).
4. Measure Absorbance of the Unknown Sample
After creating the calibration curve, measure the absorbance of your unknown sample under the same conditions (wavelength, cuvette, temperature) used for the standard solutions. Remember to subtract the blank absorbance.
5. Determine Molarity of the Unknown Sample
Using the equation of the calibration curve obtained in step 3, substitute the absorbance of the unknown sample (A) and solve for the concentration (C). This will give you the molarity of your unknown sample.
Example:
Let's say your calibration curve yields the equation: A = 1500C + 0.01. You measure the absorbance of your unknown sample to be 0.50 after subtracting the blank. Then:
0.50 = 1500C + 0.01 C = (0.50 - 0.01) / 1500 C = 3.3 x 10⁻⁴ M
Therefore, the molarity of your unknown sample is approximately 3.3 x 10⁻⁴ M.
Advanced Techniques and Considerations
Dealing with Non-Linearity
Sometimes, the relationship between absorbance and concentration deviates from linearity, especially at high concentrations. This is known as deviation from Beer-Lambert Law. In such cases:
- Dilute the sample: Diluting your sample might bring the absorbance into a linear range.
- Use a non-linear regression: If dilution is not feasible, you can use non-linear regression methods to fit the data and accurately determine the concentration.
- Consider other factors: Non-linearity can also arise from factors like chemical interactions, scattering of light, or fluorescence. Careful experimental design and data analysis are crucial.
Choosing the Appropriate Wavelength
Selecting the correct wavelength is critical for accuracy. As mentioned, using the λmax is generally preferred because it maximizes sensitivity. However, other factors like interference from other components in the solution might influence wavelength selection.
Instrumental Errors and Precision
Remember that spectrophotometry, like any analytical technique, is subject to instrumental errors. To minimize these:
- Regular calibration: Regularly calibrate your spectrophotometer using certified standards.
- Multiple measurements: Take multiple measurements of both standard solutions and the unknown sample and calculate the average to improve precision.
- Proper instrument operation: Follow the manufacturer's instructions for proper instrument operation and maintenance.
Applications of Spectrophotometry
The ability to determine molarity from absorbance has widespread applications in various fields:
- Quantitative Analysis: Determining the concentration of various substances in different samples, including pharmaceuticals, environmental pollutants, and biological molecules.
- Enzyme Kinetics: Measuring enzyme activity by monitoring the rate of substrate consumption or product formation.
- Protein Quantification: Determining the concentration of proteins using techniques like the Bradford assay or BCA assay, which rely on absorbance measurements.
- Clinical Diagnostics: Analyzing blood samples and other biological fluids to diagnose various medical conditions.
Conclusion
Determining molarity from absorbance using spectrophotometry is a fundamental technique in analytical chemistry with wide-ranging applications. By understanding the Beer-Lambert Law, preparing accurate standard solutions, creating a calibration curve, and carefully performing measurements, you can accurately determine the concentration of your analyte. Remember to always consider potential sources of error and employ appropriate techniques to improve the accuracy and precision of your results. Mastering this technique equips you with a powerful tool for quantitative analysis in various scientific endeavors.
Latest Posts
Latest Posts
-
Which Of The Following Would Not Be A Physical Change
Mar 24, 2025
-
Sample 500 Words Essay About Myself
Mar 24, 2025
-
Which Of The Following Is Vector Quantity
Mar 24, 2025
-
Gof 1 F 1 Og 1 Proof
Mar 24, 2025
-
Identify The Functional Groups In The Following Compounds
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about How To Find Molarity From Absorbance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
